Production of Viral Constructs for Neuroanatomy, Calcium Imaging, and Optogenetics
- PMID: 30883041
- PMCID: PMC6530799
- DOI: 10.1002/cpns.66
Production of Viral Constructs for Neuroanatomy, Calcium Imaging, and Optogenetics
Abstract
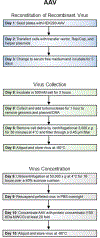
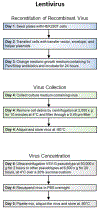
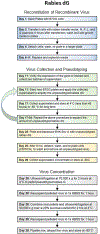
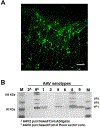
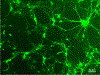
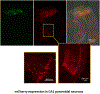
Advances in design and use of light-sensitive and light-emitting sensors have facilitated observation, measurement, and control of neuronal activities. Viruses are effective vectors for delivery of these valuable research tools to mammalian brains. Recombinant viruses are optimized to mediate regulatable, long-term, and cell-specific gene expression. Here, we describe production methods for three of the most commonly used types of recombinant viruses in neurobiology research: adeno-associated virus (AAV), retrovirus/lentivirus, and glycoprotein-deleted rabies virus. These viral constructs are frequently used for calcium imaging or to deliver neural tracers and optogenetic tools. Popular constructs are readily obtained commercially; however, customized virus production through commercial sources is time consuming and costly. This article aims to provide readers with detailed technical information for rapid production and validation of high-quality viral particles in a laboratory setting while highlighting advantages and limitations of each viral type. © 2019 by John Wiley & Sons, Inc.
Keywords: AAVs; lentivirus; rabies delta-G; recombinant virus; retrovirus; viral production.
© 2019 John Wiley & Sons, Inc.
Conflict of interest statement
CONFLICT OF INTEREST
Authors declare that they have no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

