A Comparison of Auditory Oddball Responses in Dorsolateral Prefrontal Cortex, Basolateral Amygdala, and Auditory Cortex of Macaque
- PMID: 30883292
- PMCID: PMC6995248
- DOI: 10.1162/jocn_a_01387
A Comparison of Auditory Oddball Responses in Dorsolateral Prefrontal Cortex, Basolateral Amygdala, and Auditory Cortex of Macaque
Abstract
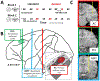
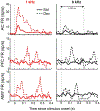
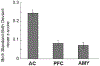
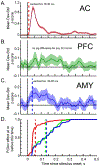
The mismatch negativity (MMN) is an ERP component seen in response to unexpected "novel" stimuli, such as in an auditory oddball task. The MMN is of wide interest and application, but the neural responses that generate it are poorly understood. This is in part due to differences in design and focus between animal and human oddball paradigms. For example, one of the main explanatory models, the "predictive error hypothesis", posits differences in timing and selectivity between signals carried in auditory and prefrontal cortex (PFC). However, these predictions have not been fully tested because (1) noninvasive techniques used in humans lack the combined spatial and temporal precision necessary for these comparisons and (2) single-neuron studies in animal models, which combine necessary spatial and temporal precision, have not focused on higher order contributions to novelty signals. In addition, accounts of the MMN traditionally do not address contributions from subcortical areas known to be involved in novelty detection, such as the amygdala. To better constrain hypotheses and to address methodological gaps between human and animal studies, we recorded single neuron activity from the auditory cortex, dorsolateral PFC, and basolateral amygdala of two macaque monkeys during an auditory oddball paradigm modeled after that used in humans. Consistent with predictions of the predictive error hypothesis, novelty signals in PFC were generally later than in auditory cortex and were abstracted from stimulus-specific effects seen in auditory cortex. However, we found signals in amygdala that were comparable in magnitude and timing to those in PFC, and both prefrontal and amygdala signals were generally much weaker than those in auditory cortex. These observations place useful quantitative constraints on putative generators of the auditory oddball-based MMN and additionally indicate that there are subcortical areas, such as the amygdala, that may be involved in novelty detection in an auditory oddball paradigm.
Conflict of interest statement
Conflict of interest: None.
Figures
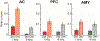


References
-
- Alain C, Woods DL, & Knight RT (1998). A distributed cortical network for auditory sensory memory in humans. Brain Research, 812(1-2), 23–37. - PubMed
-
- Alho K, Woods DL, Algazi A, Knight RT, & Naatanen R (1994). Lesions of frontal cortex diminish the auditory mismatch negativity. Electroencephalography and Clinical Neurophysiology, 91(5), 353–362. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

