The TRPP2-dependent channel of renal primary cilia also requires TRPM3
- PMID: 30883612
- PMCID: PMC6422334
- DOI: 10.1371/journal.pone.0214053
The TRPP2-dependent channel of renal primary cilia also requires TRPM3
Abstract
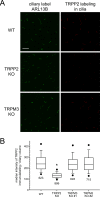
Primary cilia of renal epithelial cells express several members of the transient receptor potential (TRP) class of cation-conducting channel, including TRPC1, TRPM3, TRPM4, TRPP2, and TRPV4. Some cases of autosomal dominant polycystic kidney disease (ADPKD) are caused by defects in TRPP2 (also called polycystin-2, PC2, or PKD2). A large-conductance, TRPP2-dependent channel in renal cilia has been well described, but it is not known whether this channel includes any other protein subunits. To study this question, we investigated the pharmacology of the TRPP2-dependent channel through electrical recordings from the cilia of mIMCD-3 cells, a murine cell line of renal epithelial origin. The pharmacology was found to match that of TRPM3 channels. The ciliary TRPP2-dependent channel is known to be activated by depolarization and by increasing cytoplasmic Ca2+. This activation was greatly enhanced by external pregnenolone sulfate, an agonist of TRPM3 channels. Pregnenolone sulfate did not change the single-channel current-voltage relation. The channels were effectively blocked by isosakuranetin, a specific inhibitor of TRPM3 channels. Both pregnenolone sulfate and isosakuranetin were effective at concentrations as low as 1 μM. Knocking out TRPM3 by CRISPR/Cas9 genome editing eliminated the ciliary channel. Thus the channel is both TRPM3-dependent and TRPP2-dependent, suggesting that it may include both types of subunit. Knocking out TRPM3 did not change the level of TRPP2 protein in the cilia, so it is unlikely that the absence of functional ciliary channels results from a failure of trafficking.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
The native TRPP2-dependent channel of murine renal primary cilia.Am J Physiol Renal Physiol. 2017 Jan 1;312(1):F96-F108. doi: 10.1152/ajprenal.00272.2016. Epub 2016 Oct 19. Am J Physiol Renal Physiol. 2017. PMID: 27760766 Free PMC article.
-
A TRPM4-dependent current in murine renal primary cilia.Am J Physiol Renal Physiol. 2015 Oct 15;309(8):F697-707. doi: 10.1152/ajprenal.00294.2015. Epub 2015 Aug 19. Am J Physiol Renal Physiol. 2015. PMID: 26290373 Free PMC article.
-
Primary cilia regulate the osmotic stress response of renal epithelial cells through TRPM3.Am J Physiol Renal Physiol. 2017 Apr 1;312(4):F791-F805. doi: 10.1152/ajprenal.00465.2015. Epub 2017 Jan 25. Am J Physiol Renal Physiol. 2017. PMID: 28122715 Free PMC article.
-
TRPP2 ion channels: Critical regulators of organ morphogenesis in health and disease.Cell Calcium. 2017 Sep;66:25-32. doi: 10.1016/j.ceca.2017.05.005. Epub 2017 Jun 1. Cell Calcium. 2017. PMID: 28807147 Review.
-
TRPP2 ion channels: The roles in various subcellular locations.Biochimie. 2022 Oct;201:116-127. doi: 10.1016/j.biochi.2022.06.010. Epub 2022 Jun 26. Biochimie. 2022. PMID: 35760123 Review.
Cited by
-
TRPM channels in health and disease.Nat Rev Nephrol. 2024 Mar;20(3):175-187. doi: 10.1038/s41581-023-00777-y. Epub 2023 Oct 18. Nat Rev Nephrol. 2024. PMID: 37853091 Review.
-
Polycystins, ADPKD, and Cardiovascular Disease.Kidney Int Rep. 2019 Dec 21;5(4):396-406. doi: 10.1016/j.ekir.2019.12.007. eCollection 2020 Apr. Kidney Int Rep. 2019. PMID: 32274448 Free PMC article. Review.
-
The heteromeric PC-1/PC-2 polycystin complex is activated by the PC-1 N-terminus.Elife. 2020 Nov 9;9:e60684. doi: 10.7554/eLife.60684. Elife. 2020. PMID: 33164752 Free PMC article.
-
Regenerative Calcium Currents in Renal Primary Cilia.Front Physiol. 2022 May 10;13:894518. doi: 10.3389/fphys.2022.894518. eCollection 2022. Front Physiol. 2022. PMID: 35620606 Free PMC article.
-
Calcium signaling in polycystic kidney disease- cell death and survival.Cell Calcium. 2023 Jun;112:102733. doi: 10.1016/j.ceca.2023.102733. Epub 2023 Mar 31. Cell Calcium. 2023. PMID: 37023534 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

