Vascular endothelial growth factor increases the function of calcium-impermeable AMPA receptor GluA2 subunit in astrocytes via activation of protein kinase C signaling pathway
- PMID: 30883902
- PMCID: PMC6594043
- DOI: 10.1002/glia.23609
Vascular endothelial growth factor increases the function of calcium-impermeable AMPA receptor GluA2 subunit in astrocytes via activation of protein kinase C signaling pathway
Abstract
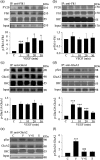
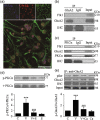
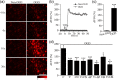
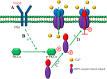
Astrocytic calcium signaling plays pivotal roles in the maintenance of neural functions and neurovascular coupling in the brain. Vascular endothelial growth factor (VEGF), an original biological substance of vessels, regulates the movement of calcium and potassium ions across neuronal membrane. In this study, we investigated whether and how VEGF regulates glutamate-induced calcium influx in astrocytes. We used cultured astrocytes combined with living cell imaging to detect the calcium influx induced by glutamate. We found that VEGF quickly inhibited the glutamate/hypoxia-induced calcium influx, which was blocked by an AMPA receptor antagonist CNQX, but not D-AP5 or UBP310, NMDA and kainate receptor antagonist, respectively. VEGF increased phosphorylation of PKCα and AMPA receptor subunit GluA2 in astrocytes, and these effects were diminished by SU1498 or calphostin C, a PKC inhibitor. With the pHluorin assay, we observed that VEGF significantly increased membrane insertion and expression of GluA2, but not GluA1, in astrocytes. Moreover, siRNA-produced knockdown of GluA2 expression in astrocytes reversed the inhibitory effect of VEGF on glutamate-induced calcium influx. Together, our results suggest that VEGF reduces glutamate-induced calcium influx in astrocytes via enhancing PKCα-mediated GluA2 phosphorylation, which in turn promotes the membrane insertion and expression of GluA2 and causes AMPA receptors to switch from calcium-permeable to calcium-impermeable receptors, thereby inhibiting astrocytic calcium influx. The present study reveals that excitatory neurotransmitter glutamate-mediated astrocytic calcium influx can be regulated by vascular biological factor via activation of AMPA receptor GluA2 subunit and uncovers a novel coupling mechanism between astrocytes and endothelial cells within the neurovascular unit.
Keywords: AMPA receptors; calcium imaging; neurovascular unit; siRNA; vascular endothelial growth factor.
© 2019 The Authors. Glia published by Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Ca2+-permeable AMPA receptors in mouse olfactory bulb astrocytes.Sci Rep. 2017 Mar 21;7:44817. doi: 10.1038/srep44817. Sci Rep. 2017. PMID: 28322255 Free PMC article.
-
Group II metabotropic glutamate receptor agonist LY379268 regulates AMPA receptor trafficking in prefrontal cortical neurons.PLoS One. 2013 Apr 12;8(4):e61787. doi: 10.1371/journal.pone.0061787. Print 2013. PLoS One. 2013. PMID: 23593498 Free PMC article.
-
Role of neuronal NR2B subunit-containing NMDA receptor-mediated Ca2+ influx and astrocytic activation in cultured mouse cortical neurons and astrocytes.Synapse. 2006 Jan;59(1):10-7. doi: 10.1002/syn.20213. Synapse. 2006. PMID: 16235228
-
GluA2-lacking, calcium-permeable AMPA receptors--inducers of plasticity?Curr Opin Neurobiol. 2011 Apr;21(2):291-8. doi: 10.1016/j.conb.2011.01.001. Epub 2011 Feb 2. Curr Opin Neurobiol. 2011. PMID: 21295464 Free PMC article. Review.
-
Subunit-specific trafficking mechanisms regulating the synaptic expression of Ca(2+)-permeable AMPA receptors.Semin Cell Dev Biol. 2014 Mar;27:14-22. doi: 10.1016/j.semcdb.2013.12.002. Epub 2013 Dec 15. Semin Cell Dev Biol. 2014. PMID: 24342448 Review.
Cited by
-
Structural prediction of GluN3 NMDA receptors.Front Physiol. 2024 Aug 20;15:1446459. doi: 10.3389/fphys.2024.1446459. eCollection 2024. Front Physiol. 2024. PMID: 39229618 Free PMC article.
-
Mapping the evolution of kainate receptor research over five decades: trends, hotspots, and emerging frontiers.Naunyn Schmiedebergs Arch Pharmacol. 2025 Aug 23. doi: 10.1007/s00210-025-04540-x. Online ahead of print. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 40848135
-
Role of vascular endothelial growth factor as a critical neurotrophic factor for the survival and physiology of motoneurons.Neural Regen Res. 2023 Aug;18(8):1691-1696. doi: 10.4103/1673-5374.363194. Neural Regen Res. 2023. PMID: 36751781 Free PMC article. Review.
-
Acute Effects of High-Intensity Aerobic Exercise on Motor Cortical Excitability and Inhibition in Sedentary Adults.Front Psychol. 2022 Mar 17;13:814633. doi: 10.3389/fpsyg.2022.814633. eCollection 2022. Front Psychol. 2022. PMID: 35369205 Free PMC article.
-
Extracellular Calcium Influx Pathways in Astrocyte Calcium Microdomain Physiology.Biomolecules. 2021 Oct 6;11(10):1467. doi: 10.3390/biom11101467. Biomolecules. 2021. PMID: 34680100 Free PMC article. Review.
References
-
- Anthony, T. E. , Klein, C. , Fishell, G. , & Heintz, N. (2004). Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron, 41(6), 881–890. - PubMed
-
- Araki, Y. , Lin, D. T. , & Huganir, R. L. (2010). Plasma membrane insertion of the AMPA receptor GluA2 subunit is regulated by NSF binding and Q/R editing of the ion pore. Proceedings of the National Academy of Sciences of the United States of America, 107(24), 11080–11085. 10.1073/pnas.1006584107 - DOI - PMC - PubMed
-
- Avraham‐Lubin, B. C. , Goldenberg‐Cohen, N. , Sadikov, T. , & Askenasy, N. (2012). VEGF induces neuroglial differentiation in bone marrow‐derived stem cells and promotes microglia conversion following mobilization with GM‐CSF. Stem Cell Reviews, 8(4), 1199–1210. 10.1007/s12015-012-9396-1 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

