Analysis of a flagellar filament cap mutant reveals that HtrA serine protease degrades unfolded flagellin protein in the periplasm of Borrelia burgdorferi
- PMID: 30883947
- PMCID: PMC6561814
- DOI: 10.1111/mmi.14243
Analysis of a flagellar filament cap mutant reveals that HtrA serine protease degrades unfolded flagellin protein in the periplasm of Borrelia burgdorferi
Abstract
Unlike external flagellated bacteria, spirochetes have periplasmic flagella (PF). Very little is known about how PF are assembled within the periplasm of spirochaetal cells. Herein, we report that FliD (BB0149), a flagellar cap protein (also named hook-associated protein 2), controls flagellin stability and flagellar filament assembly in the Lyme disease spirochete Borrelia burgdorferi. Deletion of fliD leads to non-motile mutant cells that are unable to assemble flagellar filaments and pentagon-shaped caps (10 nm in diameter, 12 nm in length). Interestingly, FlaB, a major flagellin protein of B. burgdorferi, is degraded in the fliD mutant but not in other flagella-deficient mutants (i.e., in the hook, rod, or MS-ring). Biochemical and genetic studies reveal that HtrA, a serine protease of B. burgdorferi, controls FlaB turnover. Specifically, HtrA degrades unfolded but not polymerized FlaB, and deletion of htrA increases the level of FlaB in the fliD mutant. Collectively, we propose that the flagellar cap protein FliD promotes flagellin polymerization and filament growth in the periplasm. Deletion of fliD abolishes this process, which leads to leakage of unfolded FlaB proteins into the periplasm where they are degraded by HtrA, a protease that prevents accumulation of toxic products in the periplasm.
© 2019 John Wiley & Sons Ltd.
Figures



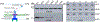
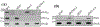
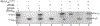

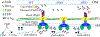
Similar articles
-
Architecture and Assembly of Periplasmic Flagellum.Microbiol Spectr. 2019 Jul;7(4):10.1128/microbiolspec.psib-0030-2019. doi: 10.1128/microbiolspec.PSIB-0030-2019. Microbiol Spectr. 2019. PMID: 31373267 Free PMC article. Review.
-
Structure and expression of the FlaA periplasmic flagellar protein of Borrelia burgdorferi.J Bacteriol. 1998 May;180(9):2418-25. doi: 10.1128/JB.180.9.2418-2425.1998. J Bacteriol. 1998. PMID: 9573194 Free PMC article.
-
A single-domain FlgJ contributes to flagellar hook and filament formation in the Lyme disease spirochete Borrelia burgdorferi.J Bacteriol. 2012 Feb;194(4):866-74. doi: 10.1128/JB.06341-11. Epub 2011 Dec 9. J Bacteriol. 2012. PMID: 22155773 Free PMC article.
-
Borrelia burgdorferi uniquely regulates its motility genes and has an intricate flagellar hook-basal body structure.J Bacteriol. 2008 Mar;190(6):1912-21. doi: 10.1128/JB.01421-07. Epub 2008 Jan 11. J Bacteriol. 2008. PMID: 18192386 Free PMC article.
-
Bacterial Flagellar Filament: A Supramolecular Multifunctional Nanostructure.Int J Mol Sci. 2021 Jul 14;22(14):7521. doi: 10.3390/ijms22147521. Int J Mol Sci. 2021. PMID: 34299141 Free PMC article. Review.
Cited by
-
Application of biophysical methods for improved protein production and characterization: A case study on an high-temperature requirement A-family bacterial protease.Protein Sci. 2022 Dec;31(12):e4498. doi: 10.1002/pro.4498. Protein Sci. 2022. PMID: 36334045 Free PMC article.
-
Architecture and Assembly of Periplasmic Flagellum.Microbiol Spectr. 2019 Jul;7(4):10.1128/microbiolspec.psib-0030-2019. doi: 10.1128/microbiolspec.PSIB-0030-2019. Microbiol Spectr. 2019. PMID: 31373267 Free PMC article. Review.
-
A Regulatory SRNA Rli43 Is Involved in the Modulation of Biofilm Formation and Virulence in Listeria monocytogenes.Pathogens. 2022 Sep 30;11(10):1137. doi: 10.3390/pathogens11101137. Pathogens. 2022. PMID: 36297193 Free PMC article.
-
Broadly conserved FlgV controls flagellar assembly and Borrelia burgdorferi dissemination in mice.bioRxiv [Preprint]. 2024 Jan 9:2024.01.09.574855. doi: 10.1101/2024.01.09.574855. bioRxiv. 2024. Update in: Nat Commun. 2024 Nov 29;15(1):10417. doi: 10.1038/s41467-024-54806-w. PMID: 38260563 Free PMC article. Updated. Preprint.
-
FlhF regulates the number and configuration of periplasmic flagella in Borrelia burgdorferi.Mol Microbiol. 2020 Jun;113(6):1122-1139. doi: 10.1111/mmi.14482. Epub 2020 Feb 21. Mol Microbiol. 2020. PMID: 32039533 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

