Effect of tetrahedral DNA nanostructures on proliferation and osteogenic differentiation of human periodontal ligament stem cells
- PMID: 30883969
- PMCID: PMC6536416
- DOI: 10.1111/cpr.12566
Effect of tetrahedral DNA nanostructures on proliferation and osteogenic differentiation of human periodontal ligament stem cells
Erratum in
-
CORRECTION.Cell Prolif. 2023 Nov;56(11):e13543. doi: 10.1111/cpr.13543. Epub 2023 Sep 13. Cell Prolif. 2023. PMID: 37705316 Free PMC article. No abstract available.
Abstract
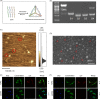
Objective: To explore the effects and underlying biological mechanisms of tetrahedral DNA nanostructures (TDNs) on the proliferation and osteogenic differentiation of periodontal ligament stem cells (PDLSCs).
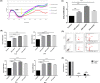
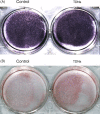
Materials and methods: Real-time cell analysis (RTCA) and CCK8 were used to screen the best concentration of TDN for PDLSCs. Cell proliferation and osteogenic differentiation were assessed after PDLSCs were treated with TDN. Data were analysed using one-way ANOVA.
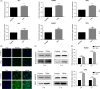
Results: Tetrahedral DNA nanostructures could play a crucial role in accelerating the proliferation of PDLSCs and had the strongest promotive effect on PDLSCs at a concentration of 250 nmol/L. Simultaneously, the osteogenic differentiation of PDLSCs could be promoted significantly by TDNs and the finding displayed that the Wnt/β-catenin signalling pathway might be the underlying biological mechanisms of TDNs on promoting the osteogenic differentiation of PDLSCs.
Conclusion: Tetrahedral DNA nanostructure treatment facilitated the proliferation of PDLSCs, significantly promoted osteogenic differentiation by regulating the Wnt/β-catenin signalling pathway. Therefore, TDNs could be a novel nanomaterial with great potential for application to PDLSC-based bone tissue engineering.
Keywords: nanomaterials; osteogenic differentiation; periodontal ligament stem cells; tetrahedral DNA nanostructure.
© 2019 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
There is no conflict of interest.
Figures


Similar articles
-
Lipopolysaccharide from Escherichia coli stimulates osteogenic differentiation of human periodontal ligament stem cells through Wnt/β-catenin-induced TAZ elevation.Mol Oral Microbiol. 2019 Feb;34(1). doi: 10.1111/omi.12249. Epub 2018 Dec 11. Mol Oral Microbiol. 2019. PMID: 30387555
-
Erythropoietin enhances osteogenic differentiation of human periodontal ligament stem cells via Wnt/β-catenin signaling pathway.Drug Des Devel Ther. 2019 Jul 26;13:2543-2552. doi: 10.2147/DDDT.S214116. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31440036 Free PMC article.
-
Osteogenic effect of crocin in human periodontal ligament stem cells via Wnt/β-catenin signaling.Oral Dis. 2024 Apr;30(3):1429-1438. doi: 10.1111/odi.14523. Epub 2023 Feb 13. Oral Dis. 2024. PMID: 36705490
-
Comparative Dental Pulp Stem Cells (DPSCs) and Periodontal Ligament Stem Cells (PDLSCs): Difference in effect of aspirin on osteoblast potential of PDLSCs and DPSCs.Tissue Cell. 2025 Jun;94:102776. doi: 10.1016/j.tice.2025.102776. Epub 2025 Feb 21. Tissue Cell. 2025. PMID: 40022908 Review.
-
The effect of the Wnt pathway on the osteogenic differentiation of periodontal ligament stem cells in different environments.PeerJ. 2025 Jan 3;13:e18770. doi: 10.7717/peerj.18770. eCollection 2025. PeerJ. 2025. PMID: 39763707 Free PMC article. Review.
Cited by
-
Advances in regenerative medicine applications of tetrahedral framework nucleic acid-based nanomaterials: an expert consensus recommendation.Int J Oral Sci. 2022 Oct 31;14(1):51. doi: 10.1038/s41368-022-00199-9. Int J Oral Sci. 2022. PMID: 36316311 Free PMC article. Review.
-
DNA hydrogels for bone regeneration.Proc Natl Acad Sci U S A. 2023 Apr 25;120(17):e2220565120. doi: 10.1073/pnas.2220565120. Epub 2023 Apr 18. Proc Natl Acad Sci U S A. 2023. PMID: 37071684 Free PMC article.
-
Nanomaterials Modulating the Fate of Dental-Derived Mesenchymal Stem Cells Involved in Oral Tissue Reconstruction: A Systematic Review.Int J Nanomedicine. 2023 Sep 21;18:5377-5406. doi: 10.2147/IJN.S418675. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37753067 Free PMC article. Review.
-
Tetrahedral framework nucleic acids ameliorate cholestatic liver disease by activating Wnt/β-catenin signaling and promoting ERK1/2 phosphorylation.Regen Biomater. 2025 Mar 20;12:rbaf017. doi: 10.1093/rb/rbaf017. eCollection 2025. Regen Biomater. 2025. PMID: 40385130 Free PMC article.
-
Effect of tetrahedral framework nucleic acids on the reconstruction of tendon-to-bone injuries after rotator cuff tears.Cell Prolif. 2024 Jun;57(6):e13605. doi: 10.1111/cpr.13605. Epub 2024 Jan 28. Cell Prolif. 2024. PMID: 38282322 Free PMC article.
References
-
- Ashman O, Phillips AM. Treatment of non‐unions with bone defects: which option and why? Injury. 2013;44(Suppl 1):S43‐S45. - PubMed
-
- Giannoudis PV, Faour O, Goff T, Kanakaris N, Dimitriou R. Masquelet technique for the treatment of bone defects: tips‐tricks and future directions. Injury. 2011;42(6):591‐598. - PubMed
-
- Brydone AS, Meek D, Maclaine S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H. 2010;224(12):1329‐1343. - PubMed
-
- Keskin D, Gundoğdu C, Atac AC . Experimental comparison of bovine‐derived xenograft, xenograft‐autologous bone marrow and autogenous bone graft for the treatment of bony defects in the rabbit ulna. Med Princ Pract. 2007;16(4):299‐305. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

