Impact of direct-acting antivirals for hepatitis C virus therapy on tacrolimus dosing in liver transplant recipients
- PMID: 30884055
- PMCID: PMC8177067
- DOI: 10.1111/tid.13078
Impact of direct-acting antivirals for hepatitis C virus therapy on tacrolimus dosing in liver transplant recipients
Abstract
Introduction: Direct-acting antivirals (DAAs) have transformed hepatitis C virus (HCV) management post-liver transplant. As HCV clears during DAA treatment, hepatic metabolism improves, resulting in decreased tacrolimus concentrations that may require dose adjustment. The purpose of this study was to determine appropriate management of immunosuppression in liver transplant recipients during and following treatment of HCV.
Methods: This study was a single-center retrospective analysis of 71 liver transplant recipients who were treated for HCV with DAAs. The primary outcome was change in dose-normalized tacrolimus concentrations from the start of DAA treatment to 12 weeks following therapy.
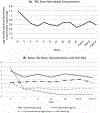
Results: The mean change in log-transformed dose-normalized tacrolimus concentrations was a reduction of 0.43 ng/mL/mg (95% CI; 0.26-0.60, P < 0.0001). The greatest decrease occurred in the first 4 weeks of treatment, after which levels stabilized. The overall mean tacrolimus concentration was 4.8 ng/mL (±2.5). Two patients (3%) developed acute cellular rejection and two patients (3%) had graft loss and died.
Conclusion: From the start of treatment to 12 weeks post-DAA therapy, liver transplant recipients experienced a significant decrease in dose-normalized tacrolimus concentrations. In conclusion, close monitoring of tacrolimus concentrations is warranted during and following treatment with DAAs, as dose increases may be indicated in order to maintain therapeutic concentrations to prevent graft rejection.
Keywords: direct-acting antiviral; hepatitis C virus; liver transplantation; tacrolimus.
© 2019 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
References
-
- Martin P, DiMartini A, Feng S, Brown R, Fallon M. Evaluation for liver transplantation in adults: 2013 practice guideline by the American Associated for the Study of Liver Diseases and the American Society of Transplantation. Hepatology. 2014;59(3):1144–1165. - PubMed
-
- Mutimer D. Pre- and post-transplant treatment of viral hepatitis C. Dig Dis. 2017;35:347–350. - PubMed
-
- Kugelmas M, Osgood MJ, Trotter JF, et al. Hepatitis C virus therapy, hepatocyte drug metabolism, and risk for acute cellular rejection. Liver Transpl. 2003;9(11):1159–1165. - PubMed
-
- Walter T, Dumortier J, Guillaud O, et al. Am J Transplant. 2007;7:177–184. - PubMed
-
- Razaee-Zacareh MS, Hesmizadeh K, Sharafi H, Alavian SM. Treatment of hepatitis C infection with direct-acting antiviral agents in liver-transplant patients: a systematic review and meta-analysis. Hepat Mon. 2017;17(6):e12324.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials