Influence of nonsynaptic α1 glycine receptors on ethanol consumption and place preference
- PMID: 30884072
- PMCID: PMC6751026
- DOI: 10.1111/adb.12726
Influence of nonsynaptic α1 glycine receptors on ethanol consumption and place preference
Abstract
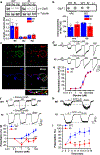
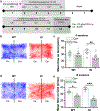
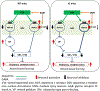
Here, we used knock-in (KI) mice that have ethanol-insensitive alpha 1 glycine receptors (GlyRs) (KK385/386AA) to examine how alpha 1 GlyRs might affect binge drinking and conditioned place preference. Data show that tonic alpha 1 GlyR-mediated currents were exclusively sensitive to ethanol only in wild-type mice. Behavioral studies showed that the KI mice have a higher intake of ethanol upon first exposure to drinking and greater conditioned place preference to ethanol. This study suggests that nonsynaptic alpha 1-containing GlyRs have a role in motivational and early reinforcing effects of ethanol.
Alcohol abuse leads to great medical, social, and economic burdens throughout the world. It is believed that the rewarding actions of alcohol are mediated by alterations in the mesolimbic dopaminergic system leading to increased levels of dopamine in the nucleus accumbens (NAc). Little is known about the role that ligand-gated ion channels (LGICs), such as glycine receptors (GlyRs), have in regulating levels of ethanol intake and place preference. In this study, we used knock-in (KI) mice that have ethanol-insensitive α1 GlyRs (KK385/386AA) and a combination of electrophysiological and behavioral approaches to examine how expression of ethanol-resistant α1 GlyRs in brain neurons might affect binge drinking and conditioned place preference. Data show that tonic α1 GlyR-mediated currents that modulate accumbal excitability were exclusively sensitive to ethanol only in wild-type (WT) mice. Behavioral studies showed that the KI mice have a higher intake of ethanol upon first exposure to drinking and greater conditioned place preference to ethanol, suggesting that α1 GlyRs in the brain have a protective role against abuse. This study suggests that nonsynaptic α1-containing GlyRs have a role in motivational and early reinforcing effects of ethanol and open a novel opportunity for pharmacotherapy development to treat alcohol use disorders.
Keywords: G-protein; alcohol and alcoholism; animal models; glycine receptor; nucleus accumbens; receptor pharmacology.
© 2019 Society for the Study of Addiction.
Figures



References
-
- Aguayo LG, Castro P, Mariqueo T, Munoz B, Xiong W, Zhang L, Lovinger DM, Homanics GE (2014) Altered sedative effects of ethanol in mice with alpha1 glycine receptor subunits that are insensitive to Gbetagamma modulation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 39:2538–2548. - PMC - PubMed
-
- Aguayo LG, Tapia JC, Pancetti FC (1996) Potentiation of the glycine-activated Cl- current by ethanol in cultured mouse spinal neurons. The Journal of pharmacology and experimental therapeutics 279:1116–1122. - PubMed
-
- Badanich KA, Mulholland PJ, Beckley JT, Trantham-Davidson H, Woodward JJ (2013) Ethanol reduces neuronal excitability of lateral orbitofrontal cortex neurons via a glycine receptor dependent mechanism. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 38:1176–1188. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

