Mice Lacking Fatty Acid-Binding Protein 5 Are Resistant to Listeria monocytogenes
- PMID: 30884482
- PMCID: PMC6758948
- DOI: 10.1159/000496405
Mice Lacking Fatty Acid-Binding Protein 5 Are Resistant to Listeria monocytogenes
Abstract
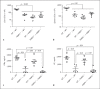
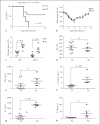
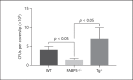
To investigate the role of fatty acid-binding protein 5 (FABP5) in infectious diseases, FABP5-deficient mice were challenged with Listeria monocytogenes, a facultative intracellular bacterial pathogen. Interestingly, FABP5-deficient animals were able to clear the infection within 3 days whereas control wild-type (WT) animals showed comparatively higher bacterial burdens in the liver and spleen. Sections of infected tissues showed an increase in inflammatory foci in WT mice compared to FABP5-deficient mice. FABP5-deficient mice had lower circulating inflammatory cytokines and increased inducible nitric oxide synthase production. FABP5-deficient mouse bone marrow-derived macrophages produced higher levels of nitrite anion than their WT counterparts in response to various stimuli. Additionally, in contrast to FABP5-/- mice, transgenic mice overexpressing FABP5 in myeloid cells (LysM-Cre driven) showed decreased survival rates and increased bacterial burden and inflammatory cytokines. Overall, these findings suggest that increased FABP5 levels correlate with a higher L. monocytogenes bacterial burden and elevated subsequent inflammation.
Keywords: FABP5; Listeria monocytogenes infection; Macrophages.
© 2019 The Author(s) Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures



References
-
- Cossart P, Toledo-Arana A. Listeria monocytogenes, a unique model in infection biology: an overview. Microbes Infect. 2008 Jul;10((9)):1041–50. - PubMed
-
- Unanue ER. Inter-relationship among macrophages, natural killer cells and neutrophils in early stages of Listeria resistance. Curr Opin Immunol. 1997 Feb;9((1)):35–43. - PubMed
-
- Shiloh MU, MacMicking JD, Nicholson S, Brause JE, Potter S, Marino M, et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999 Jan;10((1)):29–38. - PubMed
-
- Hart CM, Tolson JK, Block ER. Supplemental fatty acids alter lipid peroxidation and oxidant injury in endothelial cells. Am J Physiol. 1991 Jun;260((6 Pt 1)):L481–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

