Lack of Small Intestinal Dysbiosis Following Long-Term Selective Inhibition of Cyclooxygenase-2 by Rofecoxib in the Rat
- PMID: 30884758
- PMCID: PMC6468807
- DOI: 10.3390/cells8030251
Lack of Small Intestinal Dysbiosis Following Long-Term Selective Inhibition of Cyclooxygenase-2 by Rofecoxib in the Rat
Abstract
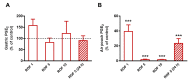
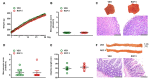
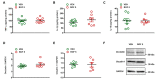
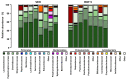
Intestinal dysbiosis is linked to numerous gastrointestinal disorders, including inflammatory bowel diseases. It is a question of debate if coxibs, selective inhibitors of cyclooxygenase (COX)-2, cause dysbiosis. Therefore, in the present study, we aimed to determine the effect of long-term (four weeks) selective inhibition of COX-2 on the small intestinal microbiota in the rat. In order to avoid mucosal damage due to topical effects and inflammation-driven microbial alterations, rofecoxib, a nonacidic compound, was used. The direct inhibitory effect of rofecoxib on the growth of bacteria was ruled out in vitro. The mucosa-sparing effect of rofecoxib was confirmed by macroscopic and histological analysis, as well as by measuring the intestinal levels of cytokines and tight junction proteins. Deep sequencing of bacterial 16S rRNA revealed that chronic rofecoxib treatment had no significant influence on the composition and diversity of jejunal microbiota. In conclusion, this is the first demonstration that long-term selective inhibition of COX-2 by rofecoxib does not cause small intestinal dysbiosis in rats. Moreover, inhibition of COX-2 activity is not likely to be responsible per se for microbial alterations caused by some coxibs, but other drug-specific properties may contribute to it.
Keywords: cyclooxygenase-2; enteropathy; inflammatory bowel diseases; intestinal dysbiosis; microbiota; rofecoxib.
Conflict of interest statement
P.F. is the founder and CEO of Pharmahungary, a group of R&D companies.
Figures
Similar articles
-
Development of intestinal, but not gastric damage caused by a low dose of indomethacin in the presence of rofecoxib.Inflammopharmacology. 2005;13(1-3):209-16. doi: 10.1163/156856005774423755. Inflammopharmacology. 2005. PMID: 16259740
-
Effect of a selective nonsteroidal anti-inflammatory inhibitor of cyclooxygenase-2 on the small bowel of rats.Braz J Med Biol Res. 2004 Mar;37(3):333-6. doi: 10.1590/s0100-879x2004000300007. Epub 2004 Mar 3. Braz J Med Biol Res. 2004. PMID: 15060699
-
Chronic treatment with rofecoxib but not ischemic preconditioning of the myocardium ameliorates early intestinal damage following cardiac ischemia/reperfusion injury in rats.Biochem Pharmacol. 2020 Aug;178:114099. doi: 10.1016/j.bcp.2020.114099. Epub 2020 Jun 12. Biochem Pharmacol. 2020. PMID: 32540483
-
Roles of COX inhibition in pathogenesis of NSAID-induced small intestinal damage.Clin Chim Acta. 2010 Apr 2;411(7-8):459-66. doi: 10.1016/j.cca.2009.12.026. Epub 2010 Jan 13. Clin Chim Acta. 2010. PMID: 20074562 Review.
-
COX-2-Specific inhibitors--the emergence of a new class of analgesic and anti-inflammatory drugs.Clin Rheumatol. 2000;19(5):331-43. doi: 10.1007/s100670070024. Clin Rheumatol. 2000. PMID: 11055820 Review.
Cited by
-
The Nonsteroidal Anti-Inflammatory Drug Ketorolac Alters the Small Intestinal Microbiota and Bile Acids Without Inducing Intestinal Damage or Delaying Peristalsis in the Rat.Front Pharmacol. 2021 Jun 4;12:664177. doi: 10.3389/fphar.2021.664177. eCollection 2021. Front Pharmacol. 2021. PMID: 34149417 Free PMC article.
-
NSAID-Gut Microbiota Interactions.Front Pharmacol. 2020 Aug 7;11:1153. doi: 10.3389/fphar.2020.01153. eCollection 2020. Front Pharmacol. 2020. PMID: 32848762 Free PMC article. Review.
-
Naproxen and prednisolone reduced intestinal alteration and permeability and bacterial translocation in rat adjuvant-induced arthritis.Inflammopharmacology. 2025 Jun;33(6):3403-3410. doi: 10.1007/s10787-025-01791-1. Epub 2025 Jun 10. Inflammopharmacology. 2025. PMID: 40493287
References
-
- Wallace J.L., Syer S., Denou E., de Palma G., Vong L., McKnight W., Jury J., Bolla M., Bercik P., Collins S.M., et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322. doi: 10.1053/j.gastro.2011.06.075. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

