Hepatitis C Direct Acting Antivirals and Ribavirin Modify Lipid but not Glucose Parameters
- PMID: 30884773
- PMCID: PMC6468811
- DOI: 10.3390/cells8030252
Hepatitis C Direct Acting Antivirals and Ribavirin Modify Lipid but not Glucose Parameters
Abstract
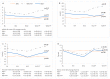
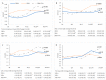
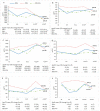
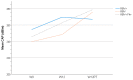
Chronic hepatitis C (HCV) infection perturbs lipid and glucose metabolism. The influenceof direct acting antiviral (DAA) treatment and ribavirin on these measures was evaluated.Furthermore, the effect of HCV cure on these parameters was assessed. Participants were allocatedto one of three 12-week treatment groups: non-cirrhotic genotype 1aparitaprevir/ritonavir/ombitasvir/dasabuvir (PrOD) plus ribavirin; non-cirrhotic 1b-PrOD;compensated cirrhotic 1a or 1b-PrOD plus ribavirin. Fasting insulin, glucose, lipid andapolipoprotein measures were assessed at baseline, Treatment Weeks 4 and 12, and 12 and 24 weekspost-dosing. Twenty-three of 24 participants achieved SVR (PP= 23/24, 96% SVR). Overall, totalcholesterol, low-density lipoprotein cholesterol (LDL-C), and triglyceride levels all increased intreatment and post-dosing. However, LDL-C levels decreased during treatment in ribavirinrecipients. Fasting glucose, insulin, and HOMA-IR were unchanged during treatment and 12 weekspost-treatment. By 12 weeks post-treatment, controlled attenuation parameter (CAP) scores, ameasure of steatosis, increased from baseline (mean 30.3 ± 63.5, p = 0.05). This regimen was safe andhighly effective and did not influence glucose metabolism. Ribavirin exposure may mitigate someon-treatment lipid changes. Further mechanistic studies are needed to understand how ribavirinimpacts lipid pathways, as there could be therapeutic implications. The metabolic pathophysiologyof increased CAP score with HCV treatment requires explanation.
Keywords: antiviral therapy; cirrhosis; insulin resistance; lipid metabolism; viral hepatitis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Baid S., Cosimi A.B., Farrell M.L., Schoenfeld D.A., Feng S., Chung R.T., Tolkoff-Rubin N., Pascual M. Posttransplant diabetes mellitus in liver transplant recipients: Risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001;72:1066–1072. doi: 10.1097/00007890-200109270-00015. - DOI - PubMed
-
- Taura N., Ichikawa T., Hamasaki K., Nakao K., Nishimura D., Goto T., Fukuta M., Kawashimo H., Fujimoto M., Kusumoto K., et al. Association between liver fibrosis and insulin sensitivity in chronic hepatitis C patients. Am. J. Gastroenterol. 2006;101:2752–2759. doi: 10.1111/j.1572-0241.2006.00835.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

