HDAC Inhibitors: Therapeutic Potential in Fibrosis-Associated Human Diseases
- PMID: 30884785
- PMCID: PMC6471162
- DOI: 10.3390/ijms20061329
HDAC Inhibitors: Therapeutic Potential in Fibrosis-Associated Human Diseases
Abstract
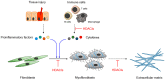
Fibrosis is characterized by excessive deposition of the extracellular matrix and develops because of fibroblast differentiation during the process of inflammation. Various cytokines stimulate resident fibroblasts, which differentiate into myofibroblasts. Myofibroblasts actively synthesize an excessive amount of extracellular matrix, which indicates pathologic fibrosis. Although initial fibrosis is a physiologic response, the accumulated fibrous material causes failure of normal organ function. Cardiac fibrosis interferes with proper diastole, whereas pulmonary fibrosis results in chronic hypoxia; liver cirrhosis induces portal hypertension, and overgrowth of fibroblasts in the conjunctiva is a major cause of glaucoma surgical failure. Recently, several reports have clearly demonstrated the functional relevance of certain types of histone deacetylases (HDACs) in various kinds of fibrosis and the successful alleviation of the condition in animal models using HDAC inhibitors. In this review, we discuss the therapeutic potential of HDAC inhibitors in fibrosis-associated human diseases using results obtained from animal models.
Keywords: HDAC; HDAC inhibitor; fibrosis; therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Inhibition of histone deacetylase 1 ameliorates renal tubulointerstitial fibrosis via modulation of inflammation and extracellular matrix gene transcription in mice.Int J Mol Med. 2018 Jan;41(1):95-106. doi: 10.3892/ijmm.2017.3218. Epub 2017 Oct 27. Int J Mol Med. 2018. PMID: 29115561 Free PMC article.
-
Aberrant expression and activity of histone deacetylases in sporadic idiopathic pulmonary fibrosis.Thorax. 2015 Nov;70(11):1022-32. doi: 10.1136/thoraxjnl-2014-206411. Epub 2015 Sep 10. Thorax. 2015. PMID: 26359372
-
Targeting cardiac fibroblasts to treat fibrosis of the heart: focus on HDACs.J Mol Cell Cardiol. 2014 May;70:100-7. doi: 10.1016/j.yjmcc.2014.02.015. Epub 2014 Mar 11. J Mol Cell Cardiol. 2014. PMID: 24631770 Free PMC article. Review.
-
Histone deacetylases in cardiac fibrosis: current perspectives for therapy.Cell Signal. 2014 Mar;26(3):521-7. doi: 10.1016/j.cellsig.2013.11.037. Epub 2013 Dec 7. Cell Signal. 2014. PMID: 24321371 Review.
-
Epigenetic modifications by histone deacetylases: Biological implications and therapeutic potential in liver fibrosis.Biochimie. 2015 Sep;116:61-9. doi: 10.1016/j.biochi.2015.06.016. Epub 2015 Jun 25. Biochimie. 2015. PMID: 26116886 Review.
Cited by
-
Targeting histone deacetylase 9 represses fibrogenic phenotypes in buccal mucosal fibroblasts with arecoline stimulation.J Dent Sci. 2024 Jan;19(1):79-85. doi: 10.1016/j.jds.2023.05.029. Epub 2023 Jun 4. J Dent Sci. 2024. PMID: 38303807 Free PMC article.
-
LP340, a novel histone deacetylase inhibitor, decreases liver injury and fibrosis in mice: role of oxidative stress and microRNA-23a.Front Pharmacol. 2024 May 17;15:1386238. doi: 10.3389/fphar.2024.1386238. eCollection 2024. Front Pharmacol. 2024. PMID: 38828459 Free PMC article.
-
Histone deacetylases: potential therapeutic targets for idiopathic pulmonary fibrosis.Front Cell Dev Biol. 2024 Aug 13;12:1426508. doi: 10.3389/fcell.2024.1426508. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39193364 Free PMC article. Review.
-
Chasing a Breath of Fresh Air in Cystic Fibrosis (CF): Therapeutic Potential of Selective HDAC6 Inhibitors to Tackle Multiple Pathways in CF Pathophysiology.J Med Chem. 2022 Feb 24;65(4):3080-3097. doi: 10.1021/acs.jmedchem.1c02067. Epub 2022 Feb 11. J Med Chem. 2022. PMID: 35148101 Free PMC article. Review.
-
Epigenetic modification mechanisms involved in keloid: current status and prospect.Clin Epigenetics. 2020 Nov 26;12(1):183. doi: 10.1186/s13148-020-00981-8. Clin Epigenetics. 2020. PMID: 33243301 Free PMC article. Review.
References
-
- Kisseleva T., Cong M., Paik Y., Scholten D., Jiang C., Benner C., Iwaisako K., Moore-Morris T., Scott B., Tsukamoto H., et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Nat. Acad. Sic. USA. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

