Non-Nutritive Sweeteners and Their Implications on the Development of Metabolic Syndrome
- PMID: 30884834
- PMCID: PMC6471792
- DOI: 10.3390/nu11030644
Non-Nutritive Sweeteners and Their Implications on the Development of Metabolic Syndrome
Abstract
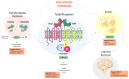
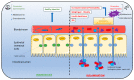
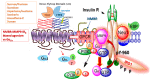
Individuals widely use non-nutritive sweeteners (NNS) in attempts to lower their overall daily caloric intake, lose weight, and sustain a healthy diet. There are insufficient scientific data that support the safety of consuming NNS. However, recent studies have suggested that NNS consumption can induce gut microbiota dysbiosis and promote glucose intolerance in healthy individuals that may result in the development of type 2 diabetes mellitus (T2DM). This sequence of events may result in changes in the gut microbiota composition through microRNA (miRNA)-mediated changes. The mechanism(s) by which miRNAs alter gene expression of different bacterial species provides a link between the consumption of NNS and the development of metabolic changes. Another potential mechanism that connects NNS to metabolic changes is the molecular crosstalk between the insulin receptor (IR) and G protein-coupled receptors (GPCRs). Here, we aim to highlight the role of NNS in obesity and discuss IR-GPCR crosstalk and miRNA-mediated changes, in the manipulation of the gut microbiota composition and T2DM pathogenesis.
Keywords: GPCR; gut microbiota; insulin receptor signaling; miRNAs; non-nutritive sweeteners; type 2 diabetes mellitus.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

