Monoterpene production by the carotenogenic yeast Rhodosporidium toruloides
- PMID: 30885220
- PMCID: PMC6421710
- DOI: 10.1186/s12934-019-1099-8
Monoterpene production by the carotenogenic yeast Rhodosporidium toruloides
Abstract
Background: Due to their high energy density and compatible physical properties, several monoterpenes have been investigated as potential renewable transportation fuels, either as blendstocks with petroleum or as drop-in replacements for use in vehicles (both heavy and light-weight) or in aviation. Sustainable microbial production of these biofuels requires the ability to utilize cheap and readily available feedstocks such as lignocellulosic biomass, which can be depolymerized into fermentable carbon sources such as glucose and xylose. However, common microbial production platforms such as the yeast Saccharomyces cerevisiae are not naturally capable of utilizing xylose, hence requiring extensive strain engineering and optimization to efficiently utilize lignocellulosic feedstocks. In contrast, the oleaginous red yeast Rhodosporidium toruloides is capable of efficiently metabolizing both xylose and glucose, suggesting that it may be a suitable host for the production of lignocellulosic bioproducts. In addition, R. toruloides naturally produces several carotenoids (C40 terpenoids), indicating that it may have a naturally high carbon flux through its mevalonate (MVA) pathway, providing pools of intermediates for the production of a wide range of heterologous terpene-based biofuels and bioproducts from lignocellulose.
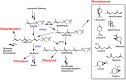
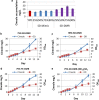
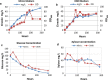
Results: Sixteen terpene synthases (TS) originating from plants, bacteria and fungi were evaluated for their ability to produce a total of nine different monoterpenes in R. toruloides. Eight of these TS were functional and produced several different monoterpenes, either as individual compounds or as mixtures, with 1,8-cineole, sabinene, ocimene, pinene, limonene, and carene being produced at the highest levels. The 1,8-cineole synthase HYP3 from Hypoxylon sp. E74060B produced the highest titer of 14.94 ± 1.84 mg/L 1,8-cineole in YPD medium and was selected for further optimization and fuel properties study. Production of 1,8-cineole from lignocellulose was also demonstrated in a 2L batch fermentation, and cineole production titers reached 34.6 mg/L in DMR-EH (Deacetylated, Mechanically Refined, Enzymatically Hydorlized) hydrolysate. Finally, the fuel properties of 1,8-cineole were examined, and indicate that it may be a suitable petroleum blend stock or drop-in replacement fuel for spark ignition engines.
Conclusion: Our results demonstrate that Rhodosporidium toruloides is a suitable microbial platform for the production of non-native monoterpenes with biofuel applications from lignocellulosic biomass.
Keywords: 1,8-Cineole; Biofuel; Monoterpene; Rhodosporidium toruloides.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Further engineering of R. toruloides for the production of terpenes from lignocellulosic biomass.Biotechnol Biofuels. 2021 Apr 21;14(1):101. doi: 10.1186/s13068-021-01950-w. Biotechnol Biofuels. 2021. PMID: 33883010 Free PMC article.
-
Rhodosporidium toruloides: a new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts.Biotechnol Biofuels. 2017 Oct 23;10:241. doi: 10.1186/s13068-017-0927-5. eCollection 2017. Biotechnol Biofuels. 2017. PMID: 29075325 Free PMC article.
-
Multiplexed CRISPR-Cas9-Based Genome Editing of Rhodosporidium toruloides.mSphere. 2019 Mar 20;4(2):e00099-19. doi: 10.1128/mSphere.00099-19. mSphere. 2019. PMID: 30894433 Free PMC article.
-
Potential of Rhodosporidium toruloides for Fatty Acids Production Using Lignocellulose Biomass.Appl Biochem Biotechnol. 2024 May;196(5):2881-2900. doi: 10.1007/s12010-023-04681-w. Epub 2023 Aug 24. Appl Biochem Biotechnol. 2024. PMID: 37615852 Review.
-
Rhodotorula toruloides: an ideal microbial cell factory to produce oleochemicals, carotenoids, and other products.World J Microbiol Biotechnol. 2021 Dec 7;38(1):13. doi: 10.1007/s11274-021-03201-4. World J Microbiol Biotechnol. 2021. PMID: 34873661 Review.
Cited by
-
Non-canonical D-xylose and L-arabinose metabolism via D-arabitol in the oleaginous yeast Rhodosporidium toruloides.Microb Cell Fact. 2023 Aug 3;22(1):145. doi: 10.1186/s12934-023-02126-x. Microb Cell Fact. 2023. PMID: 37537595 Free PMC article.
-
Further engineering of R. toruloides for the production of terpenes from lignocellulosic biomass.Biotechnol Biofuels. 2021 Apr 21;14(1):101. doi: 10.1186/s13068-021-01950-w. Biotechnol Biofuels. 2021. PMID: 33883010 Free PMC article.
-
Microbial synthesis and extraction of value-added metabolites by Rhodotorula toruloides from waste stream: a sustainable approach.Microb Cell Fact. 2025 Jun 16;24(1):134. doi: 10.1186/s12934-025-02752-7. Microb Cell Fact. 2025. PMID: 40524166 Free PMC article. Review.
-
RNA-seq analysis reveals narrow differential gene expression in MEP and MVA pathways responsible for phytochemical divergence in extreme genotypes of Thymus daenensis Celak.BMC Genomics. 2024 Mar 4;25(1):237. doi: 10.1186/s12864-024-10164-x. BMC Genomics. 2024. PMID: 38438980 Free PMC article.
-
Multi-Omics Driven Metabolic Network Reconstruction and Analysis of Lignocellulosic Carbon Utilization in Rhodosporidium toruloides.Front Bioeng Biotechnol. 2021 Jan 8;8:612832. doi: 10.3389/fbioe.2020.612832. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33585414 Free PMC article.
References
-
- Office BT. 2015 Bioenergy market report. 2017.
-
- U.S. DOE. 2016 Billion-Ton report. 2016; I:411.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

