Expansion and activation of distinct central memory T lymphocyte subsets in complex regional pain syndrome
- PMID: 30885223
- PMCID: PMC6423749
- DOI: 10.1186/s12974-019-1449-9
Expansion and activation of distinct central memory T lymphocyte subsets in complex regional pain syndrome
Erratum in
-
Correction to: Expansion and activation of distinct central memory T lymphocyte subsets in complex regional pain syndrome.J Neuroinflammation. 2019 Apr 3;16(1):70. doi: 10.1186/s12974-019-1470-z. J Neuroinflammation. 2019. PMID: 30943989 Free PMC article.
Abstract
Background: Complex regional pain syndrome (CRPS) is a debilitating condition where trauma to a limb results in devastating persistent pain that is disproportionate to the initial injury. The pathophysiology of CRPS remains unknown; however, accumulating evidence suggests it is an immunoneurological disorder, especially in light of evidence of auto-antibodies in ~ 30% of patients. Despite this, a systematic assessment of all circulating leukocyte populations in CRPS has never been performed.
Methods: We characterised 14 participants as meeting the Budapest clinical criteria for CRPS and assessed their pain ratings and psychological state using a series of questionnaires. Next, we performed immunophenotyping on blood samples from the 14 CRPS participants as well as 14 healthy pain-free controls using mass cytometry. Using a panel of 38 phenotypic and activation markers, we characterised the numbers and intracellular activation status of all major leukocyte populations using manual gating strategies and unsupervised cluster analysis.
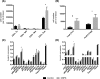
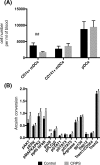
Results: We have shown expansion and activation of several distinct populations of central memory T lymphocytes in CRPS. The number of central memory CD8+ T cells was increased 2.15-fold; furthermore, this cell group had increased phosphorylation of NFkB and STAT1 compared to controls. Regarding central memory CD4+ T lymphocytes, the number of Th1 and Treg cells was increased 4.98-fold and 2.18-fold respectively, with increased phosphorylation of NFkB in both populations. We also found decreased numbers of CD1c+ myeloid dendritic cells, although with increased p38 phosphorylation. These changes could indicate dendritic cell tissue trafficking, as well as their involvement in lymphocyte activation.
Conclusions: These findings represent the first mass cytometry immunophenotyping study in any chronic pain state and provide preliminary evidence of an antigen-mediated T lymphocyte response in CRPS. In particular, the presence of increased numbers of long-lived central memory CD4+ and CD8+ T lymphocytes with increased activation of pro-inflammatory signalling pathways may indicate ongoing inflammation and cellular damage in CRPS.
Keywords: Central memory T lymphocytes; Complex regional pain syndrome; Mass cytometry; Myeloid dendritic cells; NFkB.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the University of Sydney Human Ethics committee (Approval #2017/019). Study participation was on a completely voluntary basis with all participants signed informed consent. All demographic information and blood samples were de-identified from the study team.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Elevated Plasma Levels of sIL-2R in Complex Regional Pain Syndrome: A Pathogenic Role for T-Lymphocytes?Mediators Inflamm. 2017;2017:2764261. doi: 10.1155/2017/2764261. Epub 2017 May 28. Mediators Inflamm. 2017. PMID: 28634419 Free PMC article.
-
Vitamin A deficiency alters splenic dendritic cell subsets and increases CD8(+)Gr-1(+) memory T lymphocytes in C57BL/6J mice.Cell Immunol. 2010;265(2):156-63. doi: 10.1016/j.cellimm.2010.08.006. Epub 2010 Aug 24. Cell Immunol. 2010. PMID: 20832059 Free PMC article.
-
Low blood CD8+ T-lymphocytes and high circulating monocytes are predictors of HIV-1-associated progressive encephalopathy in children.Pediatrics. 2003 Feb;111(2):E168-75. doi: 10.1542/peds.111.2.e168. Pediatrics. 2003. PMID: 12563091
-
Immunological aspects of the complex regional pain syndrome (CRPS).Curr Pharm Des. 2012;18(29):4546-9. doi: 10.2174/138161212802502206. Curr Pharm Des. 2012. PMID: 22612752 Review.
-
A brief review of complex regional pain syndrome and current management.Ann Med. 2024 Dec;56(1):2334398. doi: 10.1080/07853890.2024.2334398. Epub 2024 Apr 3. Ann Med. 2024. PMID: 38569195 Free PMC article. Review.
Cited by
-
An Integrative Review of Potential Diagnostic Biomarkers for Complex Regional Pain Syndrome.J Clin Med. 2025 May 27;14(11):3751. doi: 10.3390/jcm14113751. J Clin Med. 2025. PMID: 40507512 Free PMC article. Review.
-
Elevated circulating soluble interleukin-2 receptor (sCD25) level is associated with prefrontal excitatory-inhibitory imbalance in individuals with chronic pain: A proton MRS study.Brain Behav Immun. 2024 Aug;120:1-9. doi: 10.1016/j.bbi.2024.05.020. Epub 2024 May 19. Brain Behav Immun. 2024. PMID: 38772429 Free PMC article.
-
Serum Soluble Interleukin-2 Receptor Does Not Differentiate Complex Regional Pain Syndrome from Other Pain Conditions in a Tertiary Referral Setting.Mediators Inflamm. 2020 Sep 28;2020:6259064. doi: 10.1155/2020/6259064. eCollection 2020. Mediators Inflamm. 2020. PMID: 33061828 Free PMC article.
-
Sex differences in neuro(auto)immunity and chronic sciatic nerve pain.Biol Sex Differ. 2020 Nov 12;11(1):62. doi: 10.1186/s13293-020-00339-y. Biol Sex Differ. 2020. PMID: 33183347 Free PMC article. Review.
-
Is there an association between serum soluble interleukin-2 receptor levels and syndrome severity in persistent Complex Regional Pain Syndrome?Pain Med. 2023 Nov 2;24(11):1234-1243. doi: 10.1093/pm/pnad081. Pain Med. 2023. PMID: 37335874 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

