Hyperoxia-induced lung structure-function relation, vessel rarefaction, and cardiac hypertrophy in an infant rat model
- PMID: 30885241
- PMCID: PMC6423834
- DOI: 10.1186/s12967-019-1843-1
Hyperoxia-induced lung structure-function relation, vessel rarefaction, and cardiac hypertrophy in an infant rat model
Abstract
Background: Hyperoxia-induced bronchopulmonary dysplasia (BPD) models are essential for better understanding and impacting on long-term pulmonary, cardiovascular, and neurological sequelae of this chronic disease. Only few experimental studies have systematically compared structural alterations with lung function measurements.
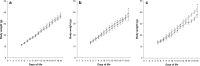
Methods: In three separate and consecutive series, Sprague-Dawley infant rats were exposed from day of life (DOL) 1 to 19 to either room air (0.21; controls) or to fractions of inspired oxygen (FiO2) of 0.6, 0.8, and 1.0. Our primary outcome parameters were histopathologic analyses of heart, lungs, and respiratory system mechanics, assessed via image analysis tools and the forced oscillation technique, respectively.
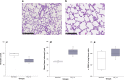
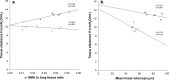
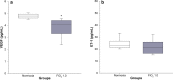
Results: Exposure to FiO2 of 0.8 and 1.0 resulted in significantly lower body weights and elevated coefficients of lung tissue damping (G) and elastance (H) when compared with controls. Hysteresivity (η) was lower due to a more pronounced increase of H when compared with G. A positive structure-function relation was demonstrated between H and the lung parenchymal content of α-smooth muscle actin (α-SMA) under hyperoxic conditions. Moreover, histology and morphometric analyses revealed alveolar simplification, fewer pulmonary arterioles, increased α-SMA content in pulmonary vessels, and right heart hypertrophy following hyperoxia. Also, in comparison to controls, hyperoxia resulted in significantly lower plasma levels of vascular endothelial growth factor (VEGF). Lastly, rats in hyperoxia showed hyperactive and a more explorative behaviour.
Conclusions: Our in vivo infant rat model mimics clinical key features of BPD. To the best of our knowledge, this is the first BPD rat model demonstrating an association between lung structure and function. Moreover, we provide additional evidence that infant rats subjected to hyperoxia develop rarefaction of pulmonary vessels, augmented vascular α-SMA, and adaptive cardiac hypertrophy. Thus, our model provides a clinically relevant tool to further investigate diseases related to O2 toxicity and to evaluate novel pharmacological treatment strategies.
Keywords: Animal model; Bronchopulmonary dysplasia; Digital pathology; Forced oscillation technique; Hyperoxia; Hysteresivity eta (η); Respiratory system mechanics; Vascular endothelial growth factor (VEGF); α-Smooth muscle actin (α-SMA).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Nintedanib preserves lung growth and prevents pulmonary hypertension in a hyperoxia-induced lung injury model.Pediatr Res. 2025 Apr;97(5):1676-1683. doi: 10.1038/s41390-024-03562-0. Epub 2024 Oct 11. Pediatr Res. 2025. PMID: 39394424 Free PMC article.
-
[Effect of intra-amniotic endotoxin priming plus hyperoxic exposure on the expression of vascular endothelial growth factor and its receptors in lungs of preterm newborn rats].Zhonghua Er Ke Za Zhi. 2007 Jul;45(7):533-8. Zhonghua Er Ke Za Zhi. 2007. PMID: 17953812 Chinese.
-
Intermittent CPAP limits hyperoxia-induced lung damage in a rabbit model of bronchopulmonary dysplasia.Am J Physiol Lung Cell Mol Physiol. 2020 May 1;318(5):L976-L987. doi: 10.1152/ajplung.00465.2019. Epub 2020 Mar 18. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32186390
-
[Role and mechanism of epithelial-mesenchymal transition in a rat model of bronchopulmonary dysplasia induced by hyperoxia exposure].Zhongguo Dang Dai Er Ke Za Zhi. 2024 Jul 15;26(7):765-773. doi: 10.7499/j.issn.1008-8830.2312112. Zhongguo Dang Dai Er Ke Za Zhi. 2024. PMID: 39014955 Free PMC article. Chinese.
-
Effects of hyperoxia on vascular tone in animal models: systematic review and meta-analysis.Crit Care. 2018 Aug 4;22(1):189. doi: 10.1186/s13054-018-2123-9. Crit Care. 2018. PMID: 30075723 Free PMC article.
Cited by
-
Hyperoxia-induced bronchopulmonary dysplasia: better models for better therapies.Dis Model Mech. 2021 Feb 23;14(2):dmm047753. doi: 10.1242/dmm.047753. Dis Model Mech. 2021. PMID: 33729989 Free PMC article. Review.
-
Single cell transcriptomic analysis of murine lung development on hyperoxia-induced damage.Nat Commun. 2021 Mar 10;12(1):1565. doi: 10.1038/s41467-021-21865-2. Nat Commun. 2021. PMID: 33692365 Free PMC article.
-
Glutamine inhibits inflammation, oxidative stress, and apoptosis and ameliorates hyperoxic lung injury.J Physiol Biochem. 2023 Aug;79(3):613-623. doi: 10.1007/s13105-023-00961-5. Epub 2023 May 5. J Physiol Biochem. 2023. PMID: 37145351
-
An Innovative Model of Bronchopulmonary Dysplasia in Premature Infants.Front Pediatr. 2020 May 27;8:271. doi: 10.3389/fped.2020.00271. eCollection 2020. Front Pediatr. 2020. PMID: 32537448 Free PMC article.
-
Characterization of circRNA-miRNA-mRNA networks regulating oxygen utilization in type II alveolar epithelial cells of Tibetan pigs.Front Mol Biosci. 2022 Sep 21;9:854250. doi: 10.3389/fmolb.2022.854250. eCollection 2022. Front Mol Biosci. 2022. PMID: 36213124 Free PMC article.
References
-
- Ridler N, Plumb J, Grocott M. Oxygen therapy in critical illness: friend or foe? A review of oxygen therapy in selected acute illnesses. J Intens Care Soc. 2014;15(3):190–198.
-
- Schmidt B. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA. 2003;289(9):1124. - PubMed
-
- Jeng S-F, Hsu C-H, Tsao P-N, Chou H-C, Lee W-T, Kao H-A, Hung H-Y, Chang J-H, Chiu N-C, Hsieh W-S. Bronchopulmonary dysplasia predicts adverse developmental and clinical outcomes in very-low-birthweight infants. Dev Med Child Neurol. 2008;50(1):51–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

