Probabilistic independent component analysis of dynamic susceptibility contrast perfusion MRI in metastatic brain tumors
- PMID: 30885275
- PMCID: PMC6423873
- DOI: 10.1186/s40644-019-0201-0
Probabilistic independent component analysis of dynamic susceptibility contrast perfusion MRI in metastatic brain tumors
Abstract
Purpose: To identify clinically relevant magnetic resonance imaging (MRI) features of different types of metastatic brain lesions, including standard anatomical, diffusion weighted imaging (DWI) and dynamic susceptibility contrast (DSC) perfusion MRI.
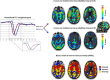
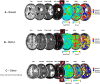
Methods: MRI imaging was retrospectively assessed on one hundred and fourteen (N = 114) brain metastases including breast (n = 27), non-small cell lung cancer (NSCLC, n = 43) and 'other' primary tumors (n = 44). Based on 114 patient's MRI scans, a total of 346 individual contrast enhancing tumors were manually segmented. In addition to tumor volume, apparent diffusion coefficients (ADC) and relative cerebral blood volume (rCBV) measurements, an independent component analysis (ICA) was performed with raw DSC data in order to assess arterio-venous components and the volume of overlap (AVOL) relative to tumor volume, as well as time to peak (TTP) of T2* signal from each component.
Results: Results suggests non-breast or non-NSCLC ('other') tumors had higher volume compare to breast and NSCLC patients (p = 0.0056 and p = 0.0003, respectively). No differences in median ADC or rCBV were observed across tumor types; however, breast and NSCLC tumors had a significantly higher "arterial" proportion of the tumor volume as indicated by ICA (p = 0.0062 and p = 0.0018, respectively), while a higher "venous" proportion were prominent in breast tumors compared with NSCLC (p = 0.0027) and 'other' lesions (p = 0.0011). The AVOL component was positively related to rCBV in all groups, but no correlation was found for arterial and venous components with respect to rCBV values. Median time to peak of arterial and venous components were 8.4 s and 12.6 s, respectively (p < 0.0001). No difference was found in arterial or venous TTP across groups.
Conclusions: Advanced ICA-derived component analysis demonstrates perfusion differences between metastatic brain tumor types that were not observable with classical ADC and rCBV measurements. These results highlight the complex relationship between brain tumor vasculature characteristics and the site of primary tumor diagnosis.
Keywords: Biomarker; Brain metastasis; Diffusion; ICA; Perfusion.
Conflict of interest statement
Ethics approval and consent to participate
This retrospective study was approved by our institutional review board (IRB) with an informed consent waiver.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Perfusion and diffusion MR imaging in enhancing malignant cerebral tumors.Eur J Radiol. 2006 Jun;58(3):394-403. doi: 10.1016/j.ejrad.2005.12.032. Epub 2006 Mar 9. Eur J Radiol. 2006. PMID: 16527438 Clinical Trial.
-
Dynamic susceptibility contrast (DSC) perfusion MRI in differential diagnosis between radionecrosis and neoangiogenesis in cerebral metastases using rCBV, rCBF and K2.Radiol Med. 2018 Jul;123(7):545-552. doi: 10.1007/s11547-018-0866-7. Epub 2018 Mar 5. Radiol Med. 2018. PMID: 29508242
-
Differentiation of Hemangioblastoma from Metastatic Brain Tumor using Dynamic Contrast-enhanced MR Imaging.Clin Neuroradiol. 2017 Sep;27(3):329-334. doi: 10.1007/s00062-016-0508-1. Epub 2016 Mar 7. Clin Neuroradiol. 2017. PMID: 26952018
-
Imaging biomarkers guided anti-angiogenic therapy for malignant gliomas.Neuroimage Clin. 2018 Jul 5;20:51-60. doi: 10.1016/j.nicl.2018.07.001. eCollection 2018. Neuroimage Clin. 2018. PMID: 30069427 Free PMC article. Review.
-
Clinical applications of dynamic susceptibility contrast perfusion-weighted MR imaging in brain tumours.Radiol Med. 2012 Apr;117(3):445-60. doi: 10.1007/s11547-011-0715-4. Epub 2011 Sep 2. Radiol Med. 2012. PMID: 21892719 Review.
Cited by
-
Multisite Benchmark Study for Standardized Relative CBV in Untreated Brain Metastases Using the DSC-MRI Consensus Acquisition Protocol.AJNR Am J Neuroradiol. 2025 Mar 4;46(3):529-535. doi: 10.3174/ajnr.A8531. AJNR Am J Neuroradiol. 2025. PMID: 39389776
-
Imaging methods to evaluate tumor microenvironment factors affecting nanoparticle drug delivery and antitumor response.Cancer Drug Resist. 2021;4(2):382-413. doi: 10.20517/cdr.2020.94. Epub 2021 Jun 19. Cancer Drug Resist. 2021. PMID: 34796317 Free PMC article.
-
Differentiation Between Glioblastoma Multiforme and Metastasis From the Lungs and Other Sites Using Combined Clinical/Routine MRI Radiomics.Front Cell Dev Biol. 2021 Aug 26;9:710461. doi: 10.3389/fcell.2021.710461. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34513840 Free PMC article.
-
Advances in the Use of Deep Learning for the Analysis of Magnetic Resonance Image in Neuro-Oncology.Cancers (Basel). 2024 Jan 10;16(2):300. doi: 10.3390/cancers16020300. Cancers (Basel). 2024. PMID: 38254790 Free PMC article. Review.
-
Fuzzy C-Means Algorithm-Based ARM-Linux-Embedded System Combined with Magnetic Resonance Imaging for Progression Prediction of Brain Tumors.Comput Math Methods Med. 2022 Mar 15;2022:4224749. doi: 10.1155/2022/4224749. eCollection 2022. Comput Math Methods Med. 2022. PMID: 35341006 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

