Rapid diagnostics for point-of-care quantification of soluble transferrin receptor
- PMID: 30885726
- PMCID: PMC6491390
- DOI: 10.1016/j.ebiom.2019.03.017
Rapid diagnostics for point-of-care quantification of soluble transferrin receptor
Abstract
Background: Iron deficiency (ID) and anaemia are major health concerns, particularly in young children. Screening for ID based on haemoglobin (Hb) concentration alone has been shown to lack sensitivity and specificity. The American Academy of Pediatrics (AAP) recommends soluble transferrin receptor (sTfR) as a promising approach to screen for iron deficiency. However, in most settings, assessment of iron status requires access to centralized laboratories. There is an urgent need for rapid, sensitive, and affordable diagnostics for sTfR at the point-of-care.
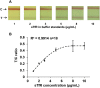
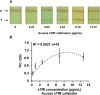
Methods: An immunochromatographic assay-based point-of-care screening device was developed for rapid quantification of sTfR from a drop of serum within a few minutes. Performance optimization of the assay was done in sTfR-spiked buffer and commercially available sTfR calibrator, followed by a small-scale proof-of-concept validation with archived serum samples.
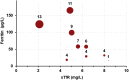
Findings: On preliminary testing with archived serum samples and comparison with Ramco ELISA, a correlation of 0.93 (P < 0.0001) was observed, demonstrating its potential for point-of-care assessment of iron status.
Interpretation: The analytical performance of the point-of-care sTfR screening device indicates the potential for application in home-use test kits and field settings, especially in low- and middle-income settings. An added advantage of sTfR quantification in combination with our previously reported serum ferritin diagnostics is in integration of Cook's equation as a quantitative and minimally-invasive indicator of total body iron stores. FUND: Thrasher Research Fund (Early Career Award #13379), NIH R03 EB 023190, NSF grant #1343058, and Nutrition International (project #10-8007-CORNE-01).
Keywords: Anaemia; Iron deficiency; Lateral flow immunoassay; Point-of-care testing; Portable diagnostics; Soluble transferrin receptor.
Copyright © 2019. Published by Elsevier B.V.
Figures



Comment in
-
The future of iron deficiency diagnostics - Rapid home-use point-of-care test kits.EBioMedicine. 2019 Apr;42:28-29. doi: 10.1016/j.ebiom.2019.03.044. Epub 2019 Mar 27. EBioMedicine. 2019. PMID: 30926425 Free PMC article. No abstract available.
References
-
- Domellof M., Dewey K.G., Lonnerdal B., Cohen R.J., Hernell O. The diagnostic criteria for iron deficiency in infants should be reevaluated. J. Nutr. 2002;132(12):3680–3686. - PubMed
-
- Baker R.D., Greer F.R. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age) Pediatrics. 2010;126(5):1040. - PubMed
-
- Skikne B.S. Serum transferrin receptor. Am. J. Hematol. 2008;83(11):872–875. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

