Antiviral Candidates for Treating Hepatitis E Virus Infection
- PMID: 30885901
- PMCID: PMC6535575
- DOI: 10.1128/AAC.00003-19
Antiviral Candidates for Treating Hepatitis E Virus Infection
Abstract
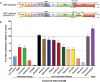
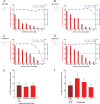
Globally, hepatitis E virus (HEV) causes significant morbidity and mortality each year. Despite this burden, there are no specific antivirals available to treat HEV patients, and the only licensed vaccine is not available outside China. Ribavirin and alpha interferon are used to treat chronic HEV infections; however, severe side effects and treatment failure are commonly reported. Therefore, this study aimed to identify potential antivirals for further development to combat HEV infection. We selected 16 compounds from the nucleoside and nonnucleoside antiviral classes that range in developmental status from late preclinical to FDA approved and evaluated them as potential antivirals for HEV infection, using genotype 1 replicon luminescence studies and replicon RNA quantification. Two potent inhibitors of HEV replication included NITD008 (half-maximal effective concentration [EC50], 0.03 μM; half-maximal cytotoxic concentration [CC50], >100 μM) and GPC-N114 (EC50, 1.07 μM, CC50, >100 μM), and both drugs reduced replicon RNA levels in cell culture (>50% reduction with either 10 μM GPC-N114 or 2.50 μM NITD008). Furthermore, GPC-N114 and NITD008 were synergistic in combinational treatment (combination index, 0.4) against HEV replication, allowing for dose reduction indices of 20.42 and 8.82 at 50% inhibition, respectively. Sofosbuvir has previously exhibited mixed results against HEV as an antiviral, both in vitro and in a few clinical applications; however, in this study it was effective against the HEV genotype 1 replicon (EC50, 1.97 μM; CC50, >100 μM) and reduced replicon RNA levels (47.2% reduction at 10 μM). Together these studies indicate drug repurposing may be a promising pathway for development of antivirals against HEV infection.
Keywords: broad-spectrum antivirals; direct-acting antivirals; hepatitis E virus; hepatitis therapy development.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- World Health Organization. 19 September 2018. Hepatitis E fact sheet. http://www.who.int/news-room/fact-sheets/detail/hepatitis-e. World Health Organization, Geneva, Switzerland: Accessed 21 October 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

