Dynamics of Organic Anion Transporter-Mediated Tubular Secretion during Postnatal Human Kidney Development and Maturation
- PMID: 30885911
- PMCID: PMC6450358
- DOI: 10.2215/CJN.10350818
Dynamics of Organic Anion Transporter-Mediated Tubular Secretion during Postnatal Human Kidney Development and Maturation
Abstract
Background and objectives: The neonatal and juvenile human kidney can be exposed to a variety of potentially toxic drugs (e.g., nonsteroidal anti-inflammatory drugs, antibiotics, antivirals, diuretics), many of which are substrates of the kidney organic anion transporters, OAT1 (SLC22A6, originally NKT) and OAT3 (SLC22A8). Despite the immense concern about the consequences of drug toxicity in this vulnerable population, the developmental regulation of OATs in the immature postnatal kidney is poorly understood.
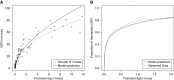
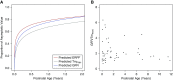
Design, setting, participants, & measurements: Recognizing that today it is difficult to obtain rich data on neonatal kidney handling of OAT probes due to technical, logistic, and ethical considerations, multiple older physiologic studies that used the prototypical organic anion substrate para-aminohippurate (PAH) were reanalyzed in order to provide a quantitative description of OAT-mediated tubular secretion across the pediatric age continuum. Parametric and semiparametric models were evaluated for kidney function outcome variables of interest (maximum tubular secretory capacity of PAH [TmPAH], effective renal plasma flow [ERPF], and GFR).
Results: Data from 119 neonates, infants, and children ranging in age from 1 day to 11.8 years were used to fit TmPAH, ERPF, and GFR as functions of postnatal age. TmPAH is low in the immediate postnatal period and increases markedly after birth, reaching 50% of the adult value (80 mg/min) at 8.3 years of age. During the first 2 years of life, TmPAH is lower than that of GFR when viewed as the fraction of the adult value.
Conclusions: During postnatal human kidney development, proximal tubule secretory function-as measured using PAH, a surrogate for OAT-mediated secretion of organic anion drugs, metabolites, and toxins-is low initially but increases rapidly. Despite developmental differences between species, this overall pattern is roughly consistent with animal studies. The human data raise the possibility that the acquisition of tubular secretory function may not closely parallel glomerular filtration.
Keywords: Adolescent; Anions; Anti-Bacterial Agents; Anti-Inflammatory Agents, Non-Steroidal; Antiviral Agents; Cephapirin; Drug-Related Side Effects and Adverse Reactions; Hippurates; Infant, Newborn; Kidney Tubules, Proximal; Organic Anion Transporters; Renal Plasma Flow, Effective; Vulnerable Populations; clinical nephrology; diuretics; drug transporter; glomerular filtration rate; kidney; kidney development; pediatric nephrology; tubular secretion.
Copyright © 2019 by the American Society of Nephrology.
Figures



Similar articles
-
Roles of organic anion transporters (OATs) in renal proximal tubules and their localization.Anat Sci Int. 2017 Mar;92(2):200-206. doi: 10.1007/s12565-016-0369-3. Epub 2016 Sep 10. Anat Sci Int. 2017. PMID: 27614971 Review.
-
Functional maturation of drug transporters in the developing, neonatal, and postnatal kidney.Mol Pharmacol. 2011 Jul;80(1):147-54. doi: 10.1124/mol.110.070680. Epub 2011 Apr 14. Mol Pharmacol. 2011. PMID: 21493727 Free PMC article.
-
Handling of Drugs, Metabolites, and Uremic Toxins by Kidney Proximal Tubule Drug Transporters.Clin J Am Soc Nephrol. 2015 Nov 6;10(11):2039-49. doi: 10.2215/CJN.02440314. Epub 2015 Oct 21. Clin J Am Soc Nephrol. 2015. PMID: 26490509 Free PMC article.
-
Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)].Kidney Int. 2005 Oct;68(4):1491-9. doi: 10.1111/j.1523-1755.2005.00612.x. Kidney Int. 2005. PMID: 16164626
-
Drug transport by Organic Anion Transporters (OATs).Pharmacol Ther. 2012 Oct;136(1):106-30. doi: 10.1016/j.pharmthera.2012.07.010. Epub 2012 Jul 25. Pharmacol Ther. 2012. PMID: 22841915 Review.
Cited by
-
Determining the Effects of Chronic Kidney Disease on Organic Anion Transporter1/3 Activity Through Physiologically Based Pharmacokinetic Modeling.Clin Pharmacokinet. 2022 Jul;61(7):997-1012. doi: 10.1007/s40262-022-01121-6. Epub 2022 May 5. Clin Pharmacokinet. 2022. PMID: 35508593
-
Contribution and expression of renal drug transporters in renal cell carcinoma.Front Pharmacol. 2025 Feb 17;15:1466877. doi: 10.3389/fphar.2024.1466877. eCollection 2024. Front Pharmacol. 2025. PMID: 40034145 Free PMC article. Review.
-
Drosophila SLC22 Orthologs Related to OATs, OCTs, and OCTNs Regulate Development and Responsiveness to Oxidative Stress.Int J Mol Sci. 2020 Mar 15;21(6):2002. doi: 10.3390/ijms21062002. Int J Mol Sci. 2020. PMID: 32183456 Free PMC article.
-
Construction and Evaluation of a Novel Organic Anion Transporter 1/3 CRISPR/Cas9 Double-Knockout Rat Model.Pharmaceutics. 2022 Oct 27;14(11):2307. doi: 10.3390/pharmaceutics14112307. Pharmaceutics. 2022. PMID: 36365126 Free PMC article.
-
Pharmacokinetics in children with chronic kidney disease.Pediatr Nephrol. 2020 Jul;35(7):1153-1172. doi: 10.1007/s00467-019-04304-9. Epub 2019 Aug 2. Pediatr Nephrol. 2020. PMID: 31375913 Free PMC article. Review.
References
-
- Toto RD: Conventional measurement of renal function utilizing serum creatinine, creatinine clearance, inulin and para-aminohippuric acid clearance. Curr Opin Nephrol Hypertens 4: 505–509, discussion 503–504, 1995 - PubMed
-
- Lepist EI, Ray AS: Beyond drug-drug interactions: Effects of transporter inhibition on endobiotics, nutrients and toxins. Expert Opin Drug Metab Toxicol 13: 1075–1087, 2017 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

