Long Noncoding RNA MPRL Promotes Mitochondrial Fission and Cisplatin Chemosensitivity via Disruption of Pre-miRNA Processing
- PMID: 30885939
- PMCID: PMC8725174
- DOI: 10.1158/1078-0432.CCR-18-2739
Long Noncoding RNA MPRL Promotes Mitochondrial Fission and Cisplatin Chemosensitivity via Disruption of Pre-miRNA Processing
Abstract
Purpose: The overall biological roles and clinical significance of most long noncoding RNAs (lncRNA) in chemosensitivity are not fully understood. We investigated the biological function, mechanism, and clinical significance of lncRNA NR_034085, which we termed miRNA processing-related lncRNA (MPRL), in tongue squamous cell carcinoma (TSCC).
Experimental design: LncRNA expression in TSCC cell lines with cisplatin treatment was measured by lncRNA microarray and confirmed in TSCC tissues. The functional roles of MPRL were demonstrated by a series of in vitro and in vivo experiments. The miRNA profiles, RNA pull-down, RNA immunoprecipitation, serial deletion analysis, and luciferase analyses were used to investigate the potential mechanisms of MPRL.
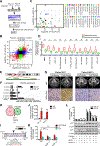
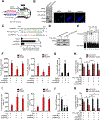
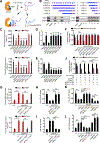
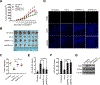
Results: We found that MPRL expression was significantly upregulated in TSCC cell lines treated with cisplatin and transactivated by E2F1. MPRL controlled mitochondrial fission and cisplatin sensitivity through miR-483-5p. In exploring the underlying interaction between MPRL and miR-483-5p, we identified that cytoplasmic MPRL directly binds to pre-miR-483 within the loop region and blocks pre-miR-483 recognition and cleavage by TRBP-DICER-complex, thereby inhibiting miR-483-5p generation and upregulating miR-483-5p downstream target-FIS1 expression. Furthermore, overexpression or knockdown MPRL altered tumor apoptosis and growth in mouse xenografts. Importantly, we found that high expression of MPRL and pre-miR-483, and low expression of miR-483-5p were significantly associated with neoadjuvant chemosensitivity and better TSCC patients' prognosis.
Conclusions: We propose a model in which lncRNAs impair microprocessor recognition and are efficient of pre-miRNA cropping. In addition, our study reveals a novel regulatory network for mitochondrial fission and chemosensitivity and new biomarkers for prediction of neoadjuvant chemosensitivity in TSCC.These findings uncover a novel mechanism by which lncRNA determines mitochondrial fission and cisplatin chemosensitivity by inhibition of pre-miRNA processing and provide for the first time the rationale for lncRNA and miRNA biogenesis for predicting chemosensitivity and patient clinical prognosis.
©2019 American Association for Cancer Research.
Conflict of interest statement
Figures


Similar articles
-
miR-483-5p determines mitochondrial fission and cisplatin sensitivity in tongue squamous cell carcinoma by targeting FIS1.Cancer Lett. 2015 Jul 1;362(2):183-91. doi: 10.1016/j.canlet.2015.03.045. Epub 2015 Apr 2. Cancer Lett. 2015. PMID: 25843291
-
Mitochondrial fission determines cisplatin sensitivity in tongue squamous cell carcinoma through the BRCA1-miR-593-5p-MFF axis.Oncotarget. 2015 Jun 20;6(17):14885-904. doi: 10.18632/oncotarget.3659. Oncotarget. 2015. PMID: 25912308 Free PMC article.
-
Actin filament-associated protein 1-antisense RNA1 promotes the development and invasion of tongue squamous cell carcinoma via the AFAP1-AS1/miR-133a-5p/ZIC2 axis.J Gene Med. 2024 Jan;26(1):e3654. doi: 10.1002/jgm.3654. J Gene Med. 2024. PMID: 38282153
-
LncRNA KCNQ1OT1 contributes to the cisplatin resistance of tongue cancer through the KCNQ1OT1/miR-124-3p/TRIM14 axis.Eur Rev Med Pharmacol Sci. 2020 Jan;24(1):200-212. doi: 10.26355/eurrev_202001_19912. Eur Rev Med Pharmacol Sci. 2020. PMID: 31957833
-
Progress in the study of long noncoding RNA in tongue squamous cell carcinoma.Oral Surg Oral Med Oral Pathol Oral Radiol. 2020 Jan;129(1):51-58. doi: 10.1016/j.oooo.2019.08.011. Epub 2019 Sep 2. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020. PMID: 31611036 Review.
Cited by
-
Non-coding RNA and drug resistance in head and neck cancer.Cancer Drug Resist. 2024 Sep 20;7:34. doi: 10.20517/cdr.2024.59. eCollection 2024. Cancer Drug Resist. 2024. PMID: 39403599 Free PMC article. Review.
-
Long Non-coding RNA LINC01787 Drives Breast Cancer Progression via Disrupting miR-125b Generation.Front Oncol. 2019 Nov 5;9:1140. doi: 10.3389/fonc.2019.01140. eCollection 2019. Front Oncol. 2019. PMID: 31750242 Free PMC article.
-
MicroRNA-483-5p Inhibits Hepatocellular Carcinoma Cell Proliferation, Cell Steatosis, and Fibrosis by Targeting PPARα and TIMP2.Cancers (Basel). 2023 Mar 10;15(6):1715. doi: 10.3390/cancers15061715. Cancers (Basel). 2023. PMID: 36980601 Free PMC article.
-
Interplay between miRNAs and lncRNAs: Mode of action and biological roles in plant development and stress adaptation.Comput Struct Biotechnol J. 2021 Apr 27;19:2567-2574. doi: 10.1016/j.csbj.2021.04.062. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34025943 Free PMC article. Review.
-
Plumbagin Enhances the Anticancer Efficacy of Cisplatin by Increasing Intracellular ROS in Human Tongue Squamous Cell Carcinoma.Oxid Med Cell Longev. 2020 Mar 25;2020:5649174. doi: 10.1155/2020/5649174. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32308804 Free PMC article.
References
-
- Zhong LP, Zhang CP, Ren GX, Guo W, William WN Jr., , Sun J, et al. Randomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinoma. J Clin Oncol. 2013;31:744–51. - PMC - PubMed
-
- Gibson MK, Li Y, Murphy B, Hussain MH, DeConti RC, Ensley J, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23:3562–7. - PubMed
-
- Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, et al. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

