The Divergent Roles of Dietary Saturated and Monounsaturated Fatty Acids on Nerve Function in Murine Models of Obesity
- PMID: 30886017
- PMCID: PMC6510336
- DOI: 10.1523/JNEUROSCI.3173-18.2019
The Divergent Roles of Dietary Saturated and Monounsaturated Fatty Acids on Nerve Function in Murine Models of Obesity
Abstract
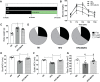
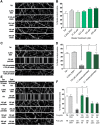
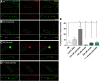
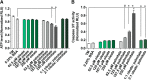
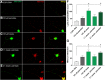
Neuropathy is the most common complication of prediabetes and diabetes and presents as distal-to-proximal loss of peripheral nerve function in the lower extremities. Neuropathy progression and disease severity in prediabetes and diabetes correlates with dyslipidemia in man and murine models of disease. Dyslipidemia is characterized by elevated levels of circulating saturated fatty acids (SFAs) that associate with the progression of neuropathy. Increased intake of monounsaturated fatty acid (MUFA)-rich diets confers metabolic health benefits; however, the impact of fatty acid saturation in neuropathy is unknown. This study examines the differential effect of SFAs and MUFAs on the development of neuropathy and the molecular mechanisms underlying the progression of the complication. Male mice Mus musculus fed a high-fat diet rich in SFAs developed robust peripheral neuropathy. This neuropathy was completely reversed by switching the mice from the SFA-rich high-fat diet to a MUFA-rich high-fat diet; nerve conduction velocities and intraepidermal nerve fiber density were restored. A MUFA oleate also prevented the impairment of mitochondrial transport and protected mitochondrial membrane potential in cultured sensory neurons treated with mixtures of oleate and the SFA palmitate. Moreover, oleate also preserved intracellular ATP levels, prevented apoptosis induced by palmitate treatment, and promoted lipid droplet formation in sensory neurons, suggesting that lipid droplets protect sensory neurons from lipotoxicity. Together, these results suggest that MUFAs reverse the progression of neuropathy by protecting mitochondrial function and transport through the formation of intracellular lipid droplets in sensory neurons.SIGNIFICANCE STATEMENT There is a global epidemic of prediabetes and diabetes, disorders that represent a continuum of metabolic disturbances in lipid and glucose metabolism. In the United States, 80 million individuals have prediabetes and 30 million have diabetes. Neuropathy is the most common complication of both disorders, carries a high morbidity, and, despite its prevalence, has no treatments. We report that dietary intervention with monounsaturated fatty acids reverses the progression of neuropathy and restores nerve function in high-fat diet-fed murine models of peripheral neuropathy. Furthermore, the addition of the monounsaturated fatty acid oleate to sensory neurons cultured under diabetic conditions shows that oleate prevents impairment of mitochondrial transport and mitochondrial dysfunction through a mechanism involving formation of axonal lipid droplets.
Keywords: diabetes; monounsaturated fatty acid; neuropathy; prediabetes; saturated fatty acid; sensory neuron.
Copyright © 2019 the authors.
Figures
References
-
- Andersen ST, Witte DR, Dalsgaard EM, Andersen H, Nawroth P, Fleming T, Jensen TM, Finnerup NB, Jensen TS, Lauritzen T, Feldman EL, Callaghan BC, Charles M (2018) Risk factors for incident diabetic polyneuropathy in a cohort with screen-detected type 2 diabetes followed for 13 years: ADDITION-denmark. Diabetes Care 41:1068–1075. 10.2337/dc17-2062 - DOI - PubMed
-
- Benador IY, Veliova M, Mahdaviani K, Petcherski A, Wikstrom JD, Assali EA, Acín-Pérez R, Shum M, Oliveira MF, Cinti S, Sztalryd C, Barshop WD, Wohlschlegel JA, Corkey BE, Liesa M, Shirihai OS (2018) Mitochondria bound to lipid droplets have unique bioenergetics, composition, and dynamics that support lipid droplet expansion. Cell Metab 27:869–885.e6. 10.1016/j.cmet.2018.03.003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
