Neural Maps of Interaural Time Difference in the American Alligator: A Stable Feature in Modern Archosaurs
- PMID: 30886018
- PMCID: PMC6520516
- DOI: 10.1523/JNEUROSCI.2989-18.2019
Neural Maps of Interaural Time Difference in the American Alligator: A Stable Feature in Modern Archosaurs
Abstract
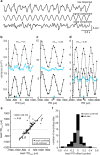
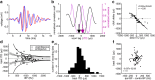
Detection of interaural time differences (ITDs) is crucial for sound localization in most vertebrates. The current view is that optimal computational strategies of ITD detection depend mainly on head size and available frequencies, although evolutionary history should also be taken into consideration. In archosaurs, which include birds and crocodiles, the brainstem nucleus laminaris (NL) developed into the critical structure for ITD detection. In birds, ITDs are mapped in an orderly array or place code, whereas in the mammalian medial superior olive, the analog of NL, maps are not found. As yet, in crocodilians, topographical representations have not been identified. However, nontopographic representations of ITD cannot be excluded due to different anatomical and ethological features of birds and crocodiles. Therefore, we measured ITD-dependent responses in the NL of anesthetized American alligators of either sex and identified the location of the recording sites by lesions made after recording. The measured extracellular field potentials, or neurophonics, were strongly ITD tuned, and their preferred ITDs correlated with the position in NL. As in birds, delay lines, which compensate for external time differences, formed maps of ITD. The broad distributions of best ITDs within narrow frequency bands were not consistent with an optimal coding model. We conclude that the available acoustic cues and the architecture of the acoustic system in early archosaurs led to a stable and similar organization in today's birds and crocodiles, although physical features, such as internally coupled ears, head size, or shape, and audible frequency range, vary among the two groups.SIGNIFICANCE STATEMENT Interaural time difference (ITD) is an important cue for sound localization, and the optimal strategies for encoding ITD in neuronal populations are the subject of ongoing debate. We show that alligators form maps of ITD very similar to birds, suggesting that their common archosaur ancestor reached a stable coding solution different from mammals. Mammals and diapsids evolved tympanic hearing independently, and local optima can be reached in evolution that are not considered by global optimal coding models. Thus, the presence of ITD maps in the brainstem may reflect a local optimum in evolutionary development. Our results underline the importance of comparative animal studies and show that optimal models must be viewed in the light of evolutionary processes.
Keywords: alligator; auditory; hearing; neurophonic; sensory; sound localization.
Copyright © 2019 the authors.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
