Targeted Degradation of Glucose Transporters Protects against Arsenic Toxicity
- PMID: 30886123
- PMCID: PMC6497993
- DOI: 10.1128/MCB.00559-18
Targeted Degradation of Glucose Transporters Protects against Arsenic Toxicity
Abstract
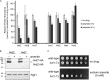
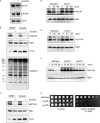
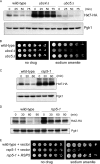
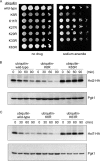
The abundance of cell surface glucose transporters must be precisely regulated to ensure optimal growth under constantly changing environmental conditions. We recently conducted a proteomic analysis of the cellular response to trivalent arsenic, a ubiquitous environmental toxin and carcinogen. A surprising finding was that a subset of glucose transporters was among the most downregulated proteins in the cell upon arsenic exposure. Here we show that this downregulation reflects targeted arsenic-dependent degradation of glucose transporters. Degradation occurs in the vacuole and requires the E2 ubiquitin ligase Ubc4, the E3 ubiquitin ligase Rsp5, and K63-linked ubiquitin chains. We used quantitative proteomic approaches to determine the ubiquitinated proteome after arsenic exposure, which helped us to identify the ubiquitination sites within these glucose transporters. A mutant lacking all seven major glucose transporters was highly resistant to arsenic, and expression of a degradation-resistant transporter restored arsenic sensitivity to this strain, suggesting that this pathway represents a protective cellular response. Previous work suggests that glucose transporters are major mediators of arsenic import, providing a potential rationale for this pathway. These results may have implications for the epidemiologic association between arsenic exposure and diabetes.
Keywords: Rsp5; arsenic; glucose transporter; protein degradation; ubiquitin.
Copyright © 2019 American Society for Microbiology.
Figures

References
-
- Maull EA, Ahsan H, Edwards J, Longnecker MP, Navas-Acien A, Pi J, Silbergeld EK, Styblo M, Tseng CH, Thayer KA, Loomis D. 2012. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 120:1658–1670. doi:10.1289/ehp.1104579. - DOI - PMC - PubMed
-
- Spratlen MJ, Grau-Perez M, Best LG, Yracheta J, Lazo M, Vaidya D, Balakrishnan P, Gamble MV, Francesconi KA, Goessler W, Cole SA, Umans JG, Howard BV, Navas-Acien A. 2018. The association of arsenic exposure and arsenic metabolism with the metabolic syndrome and its individual components: prospective evidence from the Strong Heart Family Study. Am J Epidemiol 187:1598–1612. doi:10.1093/aje/kwy048. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
