Activity and structural analysis of GRL-117C: a novel small molecule CCR5 inhibitor active against R5-tropic HIV-1s
- PMID: 30886166
- PMCID: PMC6423129
- DOI: 10.1038/s41598-019-41080-w
Activity and structural analysis of GRL-117C: a novel small molecule CCR5 inhibitor active against R5-tropic HIV-1s
Abstract
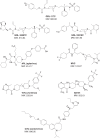
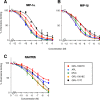
CCR5 is a member of the G-protein coupled receptor family that serves as an essential co-receptor for cellular entry of R5-tropic HIV-1, and is a validated target for therapeutics against HIV-1 infections. In the present study, we designed and synthesized a series of novel small CCR5 inhibitors and evaluated their antiviral activity. GRL-117C inhibited the replication of wild-type R5-HIV-1 with a sub-nanomolar IC50 value. These derivatives retained activity against vicriviroc-resistant HIV-1s, but did not show activity against maraviroc (MVC)-resistant HIV-1. Structural modeling indicated that the binding of compounds to CCR5 occurs in the hydrophobic cavity of CCR5 under the second extracellular loop, and amino acids critical for their binding were almost similar with those of MVC, which explains viral cross-resistance with MVC. On the other hand, one derivative, GRL-10018C, less potent against HIV-1, but more potent in inhibiting CC-chemokine binding, occupied the upper region of the binding cavity with its bis-THF moiety, presumably causing greater steric hindrance with CC-chemokines. Recent studies have shown additional unique features of certain CCR5 inhibitors such as immunomodulating properties and HIV-1 latency reversal properties, and thus, continuous efforts in developing new CCR5 inhibitors with unique binding profiles is necessary.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Synergistic Inhibition of R5 HIV-1 by the Fusion Protein (FLSC) IgG1 Fc and Maraviroc in Primary Cells: Implications for Prevention and Treatment.Curr HIV Res. 2016;14(1):24-36. doi: 10.2174/1570162x13666150909145150. Curr HIV Res. 2016. PMID: 26354735
-
The CCR5-antagonist Maraviroc reverses HIV-1 latency in vitro alone or in combination with the PKC-agonist Bryostatin-1.Sci Rep. 2017 May 24;7(1):2385. doi: 10.1038/s41598-017-02634-y. Sci Rep. 2017. PMID: 28539614 Free PMC article.
-
CCR5 antagonist TD-0680 uses a novel mechanism for enhanced potency against HIV-1 entry, cell-mediated infection, and a resistant variant.J Biol Chem. 2012 May 11;287(20):16499-509. doi: 10.1074/jbc.M112.354084. Epub 2012 Mar 23. J Biol Chem. 2012. PMID: 22447925 Free PMC article.
-
HIV co-receptors as targets for antiviral therapy.Curr Top Med Chem. 2004;4(9):883-93. doi: 10.2174/1568026043388501. Curr Top Med Chem. 2004. PMID: 15134547 Review.
-
Maraviroc: a review of its use in HIV infection and beyond.Drug Des Devel Ther. 2015 Oct 1;9:5447-68. doi: 10.2147/DDDT.S90580. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26491256 Free PMC article. Review.
Cited by
-
Targeting CCR5 as a Component of an HIV-1 Therapeutic Strategy.Front Immunol. 2022 Jan 20;12:816515. doi: 10.3389/fimmu.2021.816515. eCollection 2021. Front Immunol. 2022. PMID: 35126374 Free PMC article. Review.
-
Human TMEFF1 is a restriction factor for herpes simplex virus in the brain.Nature. 2024 Aug;632(8024):390-400. doi: 10.1038/s41586-024-07745-x. Epub 2024 Jul 24. Nature. 2024. PMID: 39048830 Free PMC article.
-
Chemokine receptor type-5: a key regulator of immunity, disease pathogenesis, and emerging therapeutic target.Inflammopharmacology. 2025 Aug;33(8):4477-4498. doi: 10.1007/s10787-025-01871-2. Epub 2025 Aug 8. Inflammopharmacology. 2025. PMID: 40779011 Review.
-
The Dual Role of CCR5 in the Course of Influenza Infection: Exploring Treatment Opportunities.Front Immunol. 2022 Jan 20;12:826621. doi: 10.3389/fimmu.2021.826621. eCollection 2021. Front Immunol. 2022. PMID: 35126379 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

