Immunotherapy in colorectal cancer: rationale, challenges and potential
- PMID: 30886395
- PMCID: PMC7295073
- DOI: 10.1038/s41575-019-0126-x
Immunotherapy in colorectal cancer: rationale, challenges and potential
Abstract
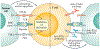
Following initial successes in melanoma treatment, immunotherapy has rapidly become established as a major treatment modality for multiple types of solid cancers, including a subset of colorectal cancers (CRCs). Two programmed cell death 1 (PD1)-blocking antibodies, pembrolizumab and nivolumab, have shown efficacy in patients with metastatic CRC that is mismatch-repair-deficient and microsatellite instability-high (dMMR-MSI-H), and have been granted accelerated FDA approval. In contrast to most other treatments for metastatic cancer, immunotherapy achieves long-term durable remission in a subset of patients, highlighting the tremendous promise of immunotherapy in treating dMMR-MSI-H metastatic CRC. Here, we review the clinical development of immune checkpoint inhibition in CRC leading to regulatory approvals for the treatment of dMMR-MSI-H CRC. We focus on new advances in expanding the efficacy of immunotherapy to early-stage CRC and CRC that is mismatch-repair-proficient and has low microsatellite instability (pMMR-MSI-L) and discuss emerging approaches for targeting the immune microenvironment, which might complement immune checkpoint inhibition.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

