Multiplexed peroxidase-based electron microscopy labeling enables simultaneous visualization of multiple cell types
- PMID: 30886406
- PMCID: PMC6555422
- DOI: 10.1038/s41593-019-0358-7
Multiplexed peroxidase-based electron microscopy labeling enables simultaneous visualization of multiple cell types
Abstract
Electron microscopy (EM) is a powerful tool for circuit mapping, but identifying specific cell types in EM datasets remains a major challenge. Here we describe a technique enabling simultaneous visualization of multiple genetically identified neuronal populations so that synaptic interactions between them can be unequivocally defined. We present 15 adeno-associated virus constructs and 6 mouse reporter lines for multiplexed EM labeling in the mammalian nervous system. These reporters feature dAPEX2, which exhibits dramatically improved signal compared with previously described ascorbate peroxidases. By targeting this enhanced peroxidase to different subcellular compartments, multiple orthogonal reporters can be simultaneously visualized and distinguished under EM using a protocol compatible with existing EM pipelines. Proof-of-principle double and triple EM labeling experiments demonstrated synaptic connections between primary afferents, descending cortical inputs, and inhibitory interneurons in the spinal cord dorsal horn. Our multiplexed peroxidase-based EM labeling system should therefore greatly facilitate analysis of connectivity in the nervous system.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures
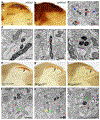




References
-
- Feinberg EH, et al. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 57, 353–363 (2008). - PubMed
Method-only References
-
- Rodriguez CI, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat. Genet 25, 139–140 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

