Lymph node conduits transport virions for rapid T cell activation
- PMID: 30886418
- PMCID: PMC6474694
- DOI: 10.1038/s41590-019-0342-0
Lymph node conduits transport virions for rapid T cell activation
Abstract
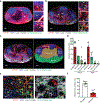
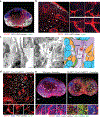
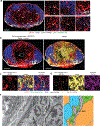
Despite intense interest in antiviral T cell priming, the routes by which virions move in lymph nodes (LNs) are imperfectly understood. Current models fail to explain how virus-infected cells rapidly appear within the LN interior after viral infection. To better understand virion trafficking in the LN, we determined the locations of virions and infected cells after administration to mice of vaccinia virus or Zika virus. Notably, many rapidly infected cells in the LN interior were adjacent to LN conduits. Through the use of confocal and electron microscopy, we clearly visualized virions within conduits. Functionally, CD8+ T cells rapidly and preferentially associated with vaccinia virus-infected cells in the LN paracortex, which led to T cell activation in the LN interior. These results reveal that it is possible for even large virions to flow through LN conduits and infect dendritic cells within the T cell zone to prime CD8+ T cells.
Conflict of interest statement
COMPETING INTERESTS
The authors declare declare no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Figures




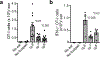
References
-
- Gretz JE, Norbury CC, Anderson AO, Proudfoot AE & Shaw S Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med 192, 1425–1440 (2000). - PMC - PubMed
-
- Itano AA et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003). - PubMed
-
- Sixt M et al. The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22, 19–29 (2005). - PubMed
-
- Pape KA, Catron DM, Itano AA & Jenkins MK The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 26, 491–502 (2007). - PubMed
-
- Gerner MY, Torabi-Parizi P & Germain RN Strategically localized dendritic cells promote rapid T cell responses to lymph-borne particulate antigens. Immunity 42, 172–185 (2015). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

