The Coming Age of Insect Cells for Manufacturing and Development of Protein Therapeutics
- PMID: 30886455
- PMCID: PMC6420222
- DOI: 10.1021/acs.iecr.8b00985
The Coming Age of Insect Cells for Manufacturing and Development of Protein Therapeutics
Abstract
Protein therapeutics is a rapidly growing segment of the pharmaceutical market. Currently, the majority of protein therapeutics are manufactured in mammalian cells for their ability to generate safe and efficacious human-like glycoproteins. The high cost of using mammalian cells for manufacturing has motivated a constant search for alternative host platforms. Insect cells have begun to emerge as a promising candidate, largely due to the development of the baculovirus expression vector system. While there are continuing efforts to improve insect-baculovirus expression for producing protein therapeutics, key limitations including cell lysis and the lack of homogeneous humanized glycosylation still remain. The field has started to see a movement toward virus-less gene expression approaches, notably the use of clustered regularly interspaced short palindromic repeats to address these shortcomings. This review highlights recent technological advances that are realizing the transformative potential of insect cells for the manufacturing and development of protein therapeutics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

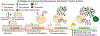
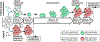
References
-
- Protein Therapeutics Market Analysis and Trends - Therapeutic Proteins, Application, Function - Forecast to 2025 https://www.researchandmarkets.com/research/6mntwc/protein (accessed Jan. 15, 2018).
-
- Leader B; Baca QJ; Golan DE Protein Therapeutics: A Summary and Pharmacological Classification. Nat. Rev. Drug Discovery 2008, 7 (1), 21. - PubMed
-
- Wen F; Rubin-Pitel SB; Zhao H Engineering of Therapeutic Proteins In Protein Engineering and Design;Park SJ, Cochran JR, Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, 2009; pp 153–177.
-
- Carter PJ Introduction to Current and Future Protein Therapeutics: A Protein Engineering Perspective. Exp. Cell Res 2011, 317 (9), 1261. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
