Short hairpin RNA-mediated knockdown of nuclear factor erythroid 2-like 3 exhibits tumor-suppressing effects in hepatocellular carcinoma cells
- PMID: 30886504
- PMCID: PMC6421239
- DOI: 10.3748/wjg.v25.i10.1210
Short hairpin RNA-mediated knockdown of nuclear factor erythroid 2-like 3 exhibits tumor-suppressing effects in hepatocellular carcinoma cells
Abstract
Background: Hepatocellular carcinoma (HCC) is one of the most common malignant tumors with high mortality-to-incidence ratios. Nuclear factor erythroid 2-like 3 (NFE2L3), also known as NRF3, is a member of the cap 'n' collar basic-region leucine zipper family of transcription factors. NFE2L3 is involved in the regulation of various biological processes, whereas its role in HCC has not been elucidated.
Aim: To explore the expression and biological function of NFE2L3 in HCC.
Methods: We analyzed the expression of NFE2L3 in HCC tissues and its correlation with clinicopathological parameters based on The Cancer Genome Atlas (TCGA) data portal. Short hairpin RNA (shRNA) interference technology was utilized to knock down NFE2L3 in vitro. Cell apoptosis, clone formation, proliferation, migration, and invasion assays were used to identify the biological effects of NFE2L3 in BEL-7404 and SMMC-7721 cells. The expression of epithelial-mesenchymal transition (EMT) markers was examined by Western blot analysis.
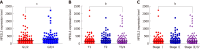
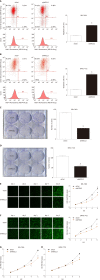
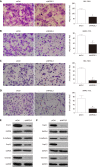
Results: TCGA analysis showed that NFE2L3 expression was significantly positively correlated with tumor grade, T stage, and pathologic stage. The qPCR and Western blot results showed that both the mRNA and protein levels of NFE2L3 were significantly decreased after shRNA-mediated knockdown in BEL-7404 and SMMC-7721 cells. The shRNA-mediated knockdown of NFE2L3 could induce apoptosis and inhibit the clone formation and cell proliferation of SMMC-7721 and BEL-7404 cells. NFE2L3 knockdown also significantly suppressed the migration, invasion, and EMT of the two cell lines.
Conclusion: Our study showed that shRNA-mediated knockdown of NFE2L3 exhibited tumor-suppressing effects in HCC cells.
Keywords: Epithelial-mesenchymal transition; Hepatocellular carcinoma; Nuclear factor erythroid 2-like 3; Short hairpin RNA; The Cancer Genome Atlas.
Conflict of interest statement
Conflict-of-interest statement: None declared.
Figures

Similar articles
-
Elevated expression of NFE2L3 promotes the development of gastric cancer through epithelial-mesenchymal transformation.Bioengineered. 2021 Dec;12(2):12204-12214. doi: 10.1080/21655979.2021.2005915. Bioengineered. 2021. PMID: 34783304 Free PMC article.
-
HCRP1 downregulation promotes hepatocellular carcinoma cell migration and invasion through the induction of EGFR activation and epithelial-mesenchymal transition.Biomed Pharmacother. 2017 Apr;88:421-429. doi: 10.1016/j.biopha.2017.01.013. Epub 2017 Jan 22. Biomed Pharmacother. 2017. PMID: 28122307
-
Long Noncoding RNA AK021443 Promotes Cell Proliferation and Migration by Regulating Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma Cells.DNA Cell Biol. 2018 May;37(5):481-490. doi: 10.1089/dna.2017.4030. Epub 2018 Mar 14. DNA Cell Biol. 2018. PMID: 29638164
-
NFE2L3 (NRF3): the Cinderella of the Cap'n'Collar transcription factors.Cell Mol Life Sci. 2011 Oct;68(20):3337-48. doi: 10.1007/s00018-011-0747-x. Epub 2011 Jun 18. Cell Mol Life Sci. 2011. PMID: 21687990 Free PMC article. Review.
-
The CNC-family transcription factor NRF3: A crucial therapeutic target for cancer treatment.Biochim Biophys Acta Mol Basis Dis. 2025 Jun;1871(5):167794. doi: 10.1016/j.bbadis.2025.167794. Epub 2025 Mar 11. Biochim Biophys Acta Mol Basis Dis. 2025. PMID: 40081618 Review.
Cited by
-
NRF3 Decreases during Melanoma Carcinogenesis and Is an Independent Prognostic Marker in Melanoma.Oxid Med Cell Longev. 2022 Mar 26;2022:2240223. doi: 10.1155/2022/2240223. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35378827 Free PMC article.
-
NFE2L3 drives hepatocellular carcinoma cell proliferation by regulating the proteasome-dependent degradation of ISGylated p53.Cancer Sci. 2023 Sep;114(9):3523-3536. doi: 10.1111/cas.15887. Epub 2023 Jun 22. Cancer Sci. 2023. PMID: 37350063 Free PMC article.
-
Multi-Omics Analysis of Molecular Characteristics and Carcinogenic Effect of NFE2L3 in Pan-Cancer.Front Genet. 2022 Jun 29;13:916973. doi: 10.3389/fgene.2022.916973. eCollection 2022. Front Genet. 2022. PMID: 35846126 Free PMC article.
-
Loss of NFE2L3 protects against inflammation-induced colorectal cancer through modulation of the tumor microenvironment.Oncogene. 2022 Mar;41(11):1563-1575. doi: 10.1038/s41388-022-02192-2. Epub 2022 Jan 28. Oncogene. 2022. PMID: 35091681 Free PMC article.
-
Roles of NRF3 in the Hallmarks of Cancer: Proteasomal Inactivation of Tumor Suppressors.Cancers (Basel). 2020 Sep 20;12(9):2681. doi: 10.3390/cancers12092681. Cancers (Basel). 2020. PMID: 32962187 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

