Utility of linked color imaging for endoscopic diagnosis of early gastric cancer
- PMID: 30886507
- PMCID: PMC6421236
- DOI: 10.3748/wjg.v25.i10.1248
Utility of linked color imaging for endoscopic diagnosis of early gastric cancer
Abstract
Background: Linked color imaging (LCI) is a method of endoscopic imaging that emphasizes slight differences in red mucosal color.
Aim: To evaluate LCI in diagnostic endoscopy of early gastric cancer and to compare LCI and pathological findings.
Methods: Endoscopic images were obtained for 39 patients (43 lesions) with early gastric cancer. Three endoscopists evaluated lesion recognition with white light imaging (WLI) and LCI. Color values in Commission Internationale de l'Eclairage (CIE) 1976 L*a*b* color space were used to calculate the color difference (ΔE) between cancer lesions and non-cancer areas. After endoscopic submucosal dissection, blood vessel density in the surface layer of the gastric epithelium was evaluated pathologically. The identical region of interest was selected for analyses of endoscopic images (WLI and LCI) and pathological analyses.
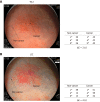
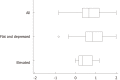
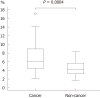
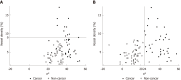
Results: LCI was superior for lesion recognition (P < 0.0001), and ΔE between cancer and non-cancer areas was significantly greater with LCI than WLI (29.4 vs 18.6, P < 0.0001). Blood vessel density was significantly higher in cancer lesions (5.96% vs 4.15%, P = 0.0004). An a* cut-off of ≥ 24 in CIE 1976 L*a*b* color space identified a cancer lesion using LCI with sensitivity of 76.7%, specificity of 93.0%, and accuracy of 84.9%.
Conclusion: LCI is more effective for recognition of early gastric cancer compared to WLI as a result of improved visualization of changes in redness. Surface blood vessel density was significantly higher in cancer lesions, and this result is consistent with LCI image analysis.
Keywords: Color difference; Early gastric cancer; Endoscopic submucosal dissection; Linked color imaging; Vessel density.
Conflict of interest statement
Conflict-of-interest statement: All authors declare no conflicts-of-interest related to this article.
Figures

Similar articles
-
Linked color imaging (LCI), a novel image-enhanced endoscopy technology, emphasizes the color of early gastric cancer.Endosc Int Open. 2017 Oct;5(10):E1005-E1013. doi: 10.1055/s-0043-117881. Epub 2017 Oct 10. Endosc Int Open. 2017. PMID: 29159276 Free PMC article.
-
Accuracies of Endoscopic Diagnosis of Helicobacter pylori-Gastritis: Multicenter Prospective Study Using White Light Imaging and Linked Color Imaging.Digestion. 2020;101(5):624-630. doi: 10.1159/000501634. Epub 2019 Jul 23. Digestion. 2020. PMID: 31336366
-
Evaluation of the visibility of early gastric cancer using linked color imaging and blue laser imaging.BMC Gastroenterol. 2017 Dec 8;17(1):150. doi: 10.1186/s12876-017-0707-5. BMC Gastroenterol. 2017. PMID: 29216843 Free PMC article.
-
Detection and characterization of early gastric cancer for curative endoscopic submucosal dissection.Dig Endosc. 2013 Mar;25 Suppl 1:44-54. doi: 10.1111/den.12004. Epub 2013 Jan 24. Dig Endosc. 2013. PMID: 23362939 Review.
-
Linked color imaging and upper gastrointestinal neoplasia.Dig Endosc. 2025 Apr;37(4):352-361. doi: 10.1111/den.14957. Epub 2024 Nov 25. Dig Endosc. 2025. PMID: 39582388 Review.
Cited by
-
Usefulness of third-generation narrow band imaging and texture and color enhancement imaging in improving visibility of superficial early gastric cancer: A study using color difference.DEN Open. 2022 Nov 24;3(1):e186. doi: 10.1002/deo2.186. eCollection 2023 Apr. DEN Open. 2022. PMID: 36439990 Free PMC article.
-
Higher detectability of gastric cancer after Helicobacter pylori eradication in texture and color enhancement imaging mode 2 in screening endoscopy.DEN Open. 2023 Jul 30;4(1):e279. doi: 10.1002/deo2.279. eCollection 2024 Apr. DEN Open. 2023. PMID: 37529380 Free PMC article.
-
Diagnostic Ability of High-definition Imaging Using Ultraslim Endoscopes in Early Gastric Cancer.J Gastric Cancer. 2021 Sep;21(3):246-257. doi: 10.5230/jgc.2021.21.e23. Epub 2021 Aug 6. J Gastric Cancer. 2021. PMID: 34691809 Free PMC article.
-
Recent advances in diagnostic upper endoscopy.World J Gastroenterol. 2020 Jan 28;26(4):433-447. doi: 10.3748/wjg.v26.i4.433. World J Gastroenterol. 2020. PMID: 32063692 Free PMC article. Review.
-
Linked Color Imaging Highlights Flat Early Gastric Cancer.Case Rep Gastroenterol. 2019 Dec 12;13(3):532-538. doi: 10.1159/000504957. eCollection 2019 Sep-Dec. Case Rep Gastroenterol. 2019. PMID: 31911767 Free PMC article.
References
-
- Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona L, Fleming T, Forouzanfar MH, Hancock J, Hay RJ, Hunter-Merrill R, Huynh C, Hosgood HD, Johnson CO, Jonas JB, Khubchandani J, Kumar GA, Kutz M, Lan Q, Larson HJ, Liang X, Lim SS, Lopez AD, MacIntyre MF, Marczak L, Marquez N, Mokdad AH, Pinho C, Pourmalek F, Salomon JA, Sanabria JR, Sandar L, Sartorius B, Schwartz SM, Shackelford KA, Shibuya K, Stanaway J, Steiner C, Sun J, Takahashi K, Vollset SE, Vos T, Wagner JA, Wang H, Westerman R, Zeeb H, Zoeckler L, Abd-Allah F, Ahmed MB, Alabed S, Alam NK, Aldhahri SF, Alem G, Alemayohu MA, Ali R, Al-Raddadi R, Amare A, Amoako Y, Artaman A, Asayesh H, Atnafu N, Awasthi A, Saleem HB, Barac A, Bedi N, Bensenor I, Berhane A, Bernabé E, Betsu B, Binagwaho A, Boneya D, Campos-Nonato I, Castañeda-Orjuela C, Catalá-López F, Chiang P, Chibueze C, Chitheer A, Choi JY, Cowie B, Damtew S, das Neves J, Dey S, Dharmaratne S, Dhillon P, Ding E, Driscoll T, Ekwueme D, Endries AY, Farvid M, Farzadfar F, Fernandes J, Fischer F, G/Hiwot TT, Gebru A, Gopalani S, Hailu A, Horino M, Horita N, Husseini A, Huybrechts I, Inoue M, Islami F, Jakovljevic M, James S, Javanbakht M, Jee SH, Kasaeian A, Kedir MS, Khader YS, Khang YH, Kim D, Leigh J, Linn S, Lunevicius R, El Razek HMA, Malekzadeh R, Malta DC, Marcenes W, Markos D, Melaku YA, Meles KG, Mendoza W, Mengiste DT, Meretoja TJ, Miller TR, Mohammad KA, Mohammadi A, Mohammed S, Moradi-Lakeh M, Nagel G, Nand D, Le Nguyen Q, Nolte S, Ogbo FA, Oladimeji KE, Oren E, Pa M, Park EK, Pereira DM, Plass D, Qorbani M, Radfar A, Rafay A, Rahman M, Rana SM, Søreide K, Satpathy M, Sawhney M, Sepanlou SG, Shaikh MA, She J, Shiue I, Shore HR, Shrime MG, So S, Soneji S, Stathopoulou V, Stroumpoulis K, Sufiyan MB, Sykes BL, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tessema GA, Thakur JS, Tran BX, Ukwaja KN, Uzochukwu BSC, Vlassov VV, Weiderpass E, Wubshet Terefe M, Yebyo HG, Yimam HH, Yonemoto N, Younis MZ, Yu C, Zaidi Z, Zaki MES, Zenebe ZM, Murray CJL, Naghavi M. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. - PMC - PubMed
-
- Hori M, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H Japan Cancer Surveillance Research Group. Cancer incidence and incidence rates in Japan in 2009: a study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2015;45:884–891. - PubMed
-
- Yao K, Yao T, Iwashita A. Determining the horizontal extent of early gastric carcinoma: two modern techniques based of differences in the mucosal microvascular architecture and density between carcinomatous and non-carcinomatous mucosa. Dig Endosc. 2002;14:83–87.
-
- Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002;56:279–284. - PubMed
-
- Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–577. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

