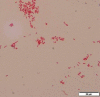
Methylobacterium infection of an arthritic knee
- PMID: 30886722
- PMCID: PMC6421341
- DOI: 10.1099/jmmcr.0.005173
Methylobacterium infection of an arthritic knee
Abstract
Introduction: Osteoarthritis (OA) is a common cause of knee pain in older adults. OA is primarily caused by deterioration of cartilage in the knee, which decreases the ability of synovial fluid to absorb shock and increases the opportunity for bones of the joint to rub together. Hylan G-F 20 (Synvisc-One) is a compound that can be injected directly into the knee to help combat the pain associated with OA by lubricating and cushioning the joint.
Case presentation: A 92-year-old male reported to his primary care provider with complaints of pain due to OA. An ultrasound-guided injection of Hylan G-F 20 was administered without complication; however, the patient presented to an emergency department approximately 10 h after the injection complaining of stabbing pain and swelling in the same knee. Specimens submitted for culture 12 h post-injection yielded a Methylobacterium spp. that was identified following biochemical testing, MALDI-TOF (matrix-assisted laser desorption/ionization-time of flight) MS analysis and bacterial sequencing. Interestingly, symptoms began to subside following aspiration of synovial fluid, and new cultures of synovial fluid collected 24 h post-Hylan G-F 20 injection were negative for the presence of Methylobacterium. The patient's knee returned to baseline with diminished pain due to OA approximately 1 week after the initial injection without antibiotic treatment.
Conclusion: We report short-term complications following treatment of OA with a Methylobacterium-contaminated lot of Hylan G-F 20.
Keywords: Hylan G-F 20; Methylobacterium; Synvisc; osteoarthritis.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures
Similar articles
-
Intra-articular viscosupplementation with hylan g-f 20 to treat osteoarthritis of the knee: an evidence-based analysis.Ont Health Technol Assess Ser. 2005;5(10):1-66. Epub 2005 Jun 1. Ont Health Technol Assess Ser. 2005. PMID: 23074461 Free PMC article.
-
Tolerability and short-term effectiveness of hylan G-F 20 in 4253 patients with osteoarthritis of the knee in clinical practice.Curr Med Res Opin. 2005 Aug;21(8):1261-9. doi: 10.1185/030079905X56501. Curr Med Res Opin. 2005. PMID: 16083536
-
Open pilot study of ultrasound-guided intra-articular injection of hylan G-F 20 (Synvisc) in the treatment of symptomatic hip osteoarthritis.Clin Rheumatol. 2005 Jun;24(3):285-9. doi: 10.1007/s10067-004-1009-1. Epub 2004 Dec 9. Clin Rheumatol. 2005. PMID: 15592903
-
Induction of an acute attack of calcium pyrophosphate dihydrate arthritis by intra-articular injection of hylan G-F 20 (Synvisc).Clin Rheumatol. 2000;19(2):147-9. doi: 10.1007/s100670050034. Clin Rheumatol. 2000. PMID: 10791628 Review.
-
The tolerability of viscosupplementation: low incidence and clinical management of local adverse events.Curr Med Res Opin. 2003;19(7):575-80. doi: 10.1185/030079903125002243. Curr Med Res Opin. 2003. PMID: 14626291 Review.
References
-
- Sanofi Genzyme . Synvisc One Prescribing Information. Cambridge, MA:: Sanofi Genzyme;; 2016. http://products.sanofi.us/synviscone/synviscone.html [accessed 23 March 2018]
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous