Aligned Ionogel Electrolytes for High-Temperature Supercapacitors
- PMID: 30886792
- PMCID: PMC6402534
- DOI: 10.1002/advs.201801337
Aligned Ionogel Electrolytes for High-Temperature Supercapacitors
Abstract
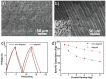
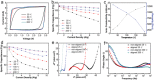
Ionogels are a new class of promising materials for use in all-solid-state energy storage devices in which they can function as an integrated separator and electrolyte. However, their performance is limited by the presence of a crosslinking polymer, which is needed to improve the mechanical properties, but compromises their ionic conductivity. Here, directional freezing is used followed by a solvent replacement method to prepare aligned nanocomposite ionogels which exhibit enhanced ionic conductivity, good mechanical strength, and thermal stability simultaneously. The aligned ionogel based supercapacitor achieves a 29% higher specific capacitance (176 F g-1 at 25 °C and 1 A g-1) than an equivalent nonaligned form. Notably, this thermally stable aligned ionogel has a high ionic conductivity of 22.1 mS cm-1 and achieves a high specific capacitance of 167 F g-1 at 10 A g-1 and 200 °C. Furthermore, the diffusion simulations conducted on 3D reconstructed tomography images are employed to explain the improved conductivity in the relevant direction of the aligned structure compared to the nonaligned. This work demonstrates the synthesis, analysis, and use of aligned ionogels as supercapacitor separators and electrolytes, representing a promising direction for the development of wearable electronics coupled with image based process and simulations.
Keywords: X‐ray tomography; aligned ionogels; supercapacitors; thermal tolerance; tortuosity factors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- a) Ding Y., Zhang J., Chang L., Zhang X., Liu H., Jiang L., Adv. Mater. 2017, 29, 1; - PubMed
- b) Le Bideau J., Viau L., Vioux A., Chem. Soc. Rev. 2011, 40, 907; - PubMed
- c) Chen N., Zhang H., Li L., Chen R., Guo S., Adv. Energy Mater. 2018, 8, 1;
- d) Cheng X., Pan J., Zhao Y., Liao M., Peng H., Adv. Energy Mater. 2018, 8, 1;
- e) Shi Y., Zhang J., Pan L., Shi Y., Yu G., Nano Today 2016, 11, 738.
-
- a) Chen N., Dai Y., Xing Y., Wang L., Guo C., Chen R., Guo S., Wu F., Energy Environ. Sci. 2017, 10, 1660;
- b) Wu F., Chen N., Chen R., Wang L., Li L., Nano Energy 2017, 31, 9.
-
- a) Zhang X., Kar M., Mendes T. C., Wu Y., Macfarlane D. R., Adv. Energy Mater. 2018, 1, 1702702;
- b) Liu X., Wu D., Wang H., Wang Q., Adv. Mater. 2014, 26, 4370; - PubMed
- c) Liu X., Wu B., Brandon N., Wang Q., Energy Technol. 2017, 5, 220;
- d) Dou Q., Liu L., Yang B., Lang J., Yan X., Nat. Commun. 2017, 8. - PMC - PubMed
-
- Eustache E., Douard C., Demortière A., De Andrade V., Brachet M., Le Bideau J., Brousse T., Lethien C., Adv. Mater. Technol. 2017, 2, 1700126.
-
- Miran M. S., Yasuda T., Susan M. A. B. H., Dokko K., Watanabe M., J. Phys. Chem. C 2014, 118, 27631.
LinkOut - more resources
Full Text Sources
