Synergistic MicroRNA Therapy in Liver Fibrotic Rat Using MRI-Visible Nanocarrier Targeting Hepatic Stellate Cells
- PMID: 30886803
- PMCID: PMC6402399
- DOI: 10.1002/advs.201801809
Synergistic MicroRNA Therapy in Liver Fibrotic Rat Using MRI-Visible Nanocarrier Targeting Hepatic Stellate Cells
Abstract
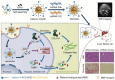
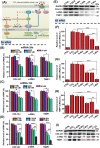
Liver fibrosis, as one of the leading causes of liver-related morbidity and mortality, has no Food and Drug Administration (FDA)-approved antifibrotic therapy yet. Although microRNA-29b (miRNA-29b) and microRNA-122 (miRNA-122) have great potential in treating liver fibrosis via regulating profibrotic genes in hepatic stellate cells (HSCs), it is still a challenge to achieve a HSC-targeted and meanwhile noninvasively trackable delivery of miRNAs in vivo. Herein, a pH-sensitive and vitamin A (VA)-conjugated copolymer VA-polyethylene glycol-polyethyleneimine-poly(N-(N',N'-diisopropylaminoethyl)-co-benzylamino) aspartamide (T-PBP) is synthesized and assembled into superparamagnetic iron oxide (SPIO)-decorated cationic micelle for miRNA delivery. The T-PBP micelle efficiently transports the miRNA-29b and miRNA-122 to HSC in a magnetic resonance imaging-visible manner, resulting in a synergistic antifibrosis effect via downregulating the expression of fibrosis-related genes, including collagen type I alpha 1, α-smooth muscle actin, and tissue inhibitor of metalloproteinase 1. Consequently, the HSC-targeted combination therapy with miRNA-29b and miRNA-122 demonstrates a prominent antifibrotic efficacy in terms of improving liver function and relieving hepatic fibrosis.
Keywords: hepatic stellate cells; liver fibrosis; magnetic resonance imaging visibility; miRNA delivery; nanomedicine; synergistic therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Selective inhibition of glycolysis in hepatic stellate cells and suppression of liver fibrogenesis with vitamin A-derivative decorated camptothecin micelles.Acta Biomater. 2023 Sep 15;168:497-514. doi: 10.1016/j.actbio.2023.07.035. Epub 2023 Jul 26. Acta Biomater. 2023. PMID: 37507035
-
microRNA-29b prevents liver fibrosis by attenuating hepatic stellate cell activation and inducing apoptosis through targeting PI3K/AKT pathway.Oncotarget. 2015 Mar 30;6(9):7325-38. doi: 10.18632/oncotarget.2621. Oncotarget. 2015. PMID: 25356754 Free PMC article.
-
Collagenase-I decorated co-delivery micelles potentiate extracellular matrix degradation and hepatic stellate cell targeting for liver fibrosis therapy.Acta Biomater. 2022 Oct 15;152:235-254. doi: 10.1016/j.actbio.2022.08.065. Epub 2022 Sep 8. Acta Biomater. 2022. PMID: 36087869
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
The roles of microRNA families in hepatic fibrosis.Cell Biosci. 2017 Jul 4;7:34. doi: 10.1186/s13578-017-0161-7. eCollection 2017. Cell Biosci. 2017. PMID: 28680559 Free PMC article. Review.
Cited by
-
Polymeric nanomedicines for the treatment of hepatic diseases.J Nanobiotechnology. 2022 Nov 19;20(1):488. doi: 10.1186/s12951-022-01708-y. J Nanobiotechnology. 2022. PMID: 36402994 Free PMC article. Review.
-
An Artificial Intelligent Signal Amplification System for in vivo Detection of miRNA.Front Bioeng Biotechnol. 2019 Nov 21;7:330. doi: 10.3389/fbioe.2019.00330. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31824932 Free PMC article.
-
Stimuli-Responsive Nanotechnology for RNA Delivery.Adv Sci (Weinh). 2023 Dec;10(36):e2303597. doi: 10.1002/advs.202303597. Epub 2023 Nov 1. Adv Sci (Weinh). 2023. PMID: 37915127 Free PMC article. Review.
-
Bimodal Imaging-Visible Nanomedicine Integrating CXCR4 and VEGFa Genes Directs Synergistic Reendothelialization of Endothelial Progenitor Cells.Adv Sci (Weinh). 2020 Nov 9;7(24):2001657. doi: 10.1002/advs.202001657. eCollection 2020 Dec. Adv Sci (Weinh). 2020. PMID: 33344118 Free PMC article.
-
Enhancing the Efficacy of Metal-Free MRI Contrast Agents via Conjugating Nitroxides onto PEGylated Cross-Linked Poly(Carboxylate Ester).Adv Sci (Weinh). 2020 Jun 3;7(14):2000467. doi: 10.1002/advs.202000467. eCollection 2020 Jul. Adv Sci (Weinh). 2020. PMID: 32714757 Free PMC article.
References
-
- Vogt J., Traynor R., Sapkota G. P., Cell. Signalling 2011, 23, 1831. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
