Synergistic MicroRNA Therapy in Liver Fibrotic Rat Using MRI-Visible Nanocarrier Targeting Hepatic Stellate Cells
- PMID: 30886803
- PMCID: PMC6402399
- DOI: 10.1002/advs.201801809
Synergistic MicroRNA Therapy in Liver Fibrotic Rat Using MRI-Visible Nanocarrier Targeting Hepatic Stellate Cells
Abstract
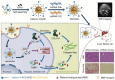
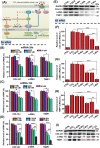
Liver fibrosis, as one of the leading causes of liver-related morbidity and mortality, has no Food and Drug Administration (FDA)-approved antifibrotic therapy yet. Although microRNA-29b (miRNA-29b) and microRNA-122 (miRNA-122) have great potential in treating liver fibrosis via regulating profibrotic genes in hepatic stellate cells (HSCs), it is still a challenge to achieve a HSC-targeted and meanwhile noninvasively trackable delivery of miRNAs in vivo. Herein, a pH-sensitive and vitamin A (VA)-conjugated copolymer VA-polyethylene glycol-polyethyleneimine-poly(N-(N',N'-diisopropylaminoethyl)-co-benzylamino) aspartamide (T-PBP) is synthesized and assembled into superparamagnetic iron oxide (SPIO)-decorated cationic micelle for miRNA delivery. The T-PBP micelle efficiently transports the miRNA-29b and miRNA-122 to HSC in a magnetic resonance imaging-visible manner, resulting in a synergistic antifibrosis effect via downregulating the expression of fibrosis-related genes, including collagen type I alpha 1, α-smooth muscle actin, and tissue inhibitor of metalloproteinase 1. Consequently, the HSC-targeted combination therapy with miRNA-29b and miRNA-122 demonstrates a prominent antifibrotic efficacy in terms of improving liver function and relieving hepatic fibrosis.
Keywords: hepatic stellate cells; liver fibrosis; magnetic resonance imaging visibility; miRNA delivery; nanomedicine; synergistic therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Vogt J., Traynor R., Sapkota G. P., Cell. Signalling 2011, 23, 1831. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
