How Viral and Intracellular Bacterial Pathogens Reprogram the Metabolism of Host Cells to Allow Their Intracellular Replication
- PMID: 30886834
- PMCID: PMC6409310
- DOI: 10.3389/fcimb.2019.00042
How Viral and Intracellular Bacterial Pathogens Reprogram the Metabolism of Host Cells to Allow Their Intracellular Replication
Abstract
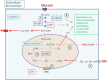
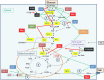
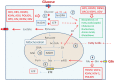
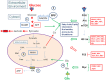
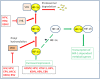
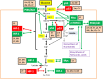
Viruses and intracellular bacterial pathogens (IBPs) have in common the need of suitable host cells for efficient replication and proliferation during infection. In human infections, the cell types which both groups of pathogens are using as hosts are indeed quite similar and include phagocytic immune cells, especially monocytes/macrophages (MOs/MPs) and dendritic cells (DCs), as well as nonprofessional phagocytes, like epithelial cells, fibroblasts and endothelial cells. These terminally differentiated cells are normally in a metabolically quiescent state when they are encountered by these pathogens during infection. This metabolic state of the host cells does not meet the extensive need for nutrients required for efficient intracellular replication of viruses and especially IBPs which, in contrast to the viral pathogens, have to perform their own specific intracellular metabolism to survive and efficiently replicate in their host cell niches. For this goal, viruses and IBPs have to reprogram the host cell metabolism in a pathogen-specific manner to increase the supply of nutrients, energy, and metabolites which have to be provided to the pathogen to allow its replication. In viral infections, this appears to be often achieved by the interaction of specific viral factors with central metabolic regulators, including oncogenes and tumor suppressors, or by the introduction of virus-specific oncogenes. Less is so far known on the mechanisms leading to metabolic reprogramming of the host cell by IBPs. However, the still scant data suggest that similar mechanisms may also determine the reprogramming of the host cell metabolism in IBP infections. In this review, we summarize and compare the present knowledge on this important, yet still poorly understood aspect of pathogenesis of human viral and especially IBP infections.
Keywords: intracellular bacterial pathogens; metabolic adaptation; metabolism of infected and uninfected host cells; reprogamming of host cell metabolism; viruses.
Figures
References
-
- Adams O., Besken K., Oberdorfer C., Mackenzie C. R., Takikawa O., Daubener W. (2004). Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J. Virol. 78, 2632–2636. 10.1128/JVI.78.5.2632-2636.2004 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

