Treatment of patients with recurrent epithelial ovarian cancer for whom platinum is still an option
- PMID: 30887020
- PMCID: PMC8887593
- DOI: 10.1093/annonc/mdz104
Treatment of patients with recurrent epithelial ovarian cancer for whom platinum is still an option
Abstract
Background: Ovarian cancer remains the most deadly gynecologic cancer with the majority of patients relapsing within 3 years of diagnosis. Traditional treatment paradigms linked to platinum sensitivity or resistance are currently being questioned in the setting of new diagnostic methods and treatment options.
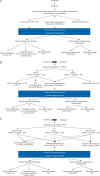
Design: Authors carried out review of the literature on key topics in treatment of recurrent epithelial ovarian cancer (EOC) when platinum is still an option; including secondary surgical cytoreduction, chemotherapy, novel treatment options, and maintenance therapy. A treatment algorithm is proposed.
Results: Molecular characterization of EOC is critical to help guide treatment decisions. The role of secondary cytoreductive surgery is currently being evaluated with results from Gynecologic Oncology Group (GOG) 213 and anticipated results from DESKTOP III clinical trials. Chemotherapy backbone has remained relatively unchanged but utilizing non-platinum-based regimens is under investigation. In addition, maintenance therapy with anti-angiogenic therapy and Poly (ADP-ribose) Polymerase (PARP) inhibitors has emerged as the standard of care. Novel combinations, including immunotherapy and anti-angiogenesis agents, may further change the current landscape.
Conclusions: The treatment of recurrent EOC is rapidly changing. Clinical trial design will need to continue to evolve as many novel therapies move to the upfront setting. Ultimately, the treatment of patients with recurrent EOC must incorporate individual patient and tumor factors.
Keywords: maintenance therapy; molecular characterization; ovarian cancer; platinum.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- American Cancer Society. Cancer Facts and Figures 2018. Atlanta, GA 2018. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts....
-
- Alvarez RD, Matulonis UA, Herzog TJ. et al. Moving beyond the platinum sensitive/resistant paradigm for patients with recurrent ovarian cancer. Gynecol Oncol 2016; 141(3): 405–409. - PubMed
-
- Chi DS, McCaughty K, Diaz JP. et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 2006; 106(9): 1933–1939. - PubMed
-
- Harter P, du Bois A, Hahmann M. et al. Surgery in recurrent ovarian cancer: the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann Surg Oncol 2006; 13(12): 1702–1710. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

