Protective effects and mechanisms of omega-3 polyunsaturated fatty acid on intestinal injury and macrophage polarization in peritoneal dialysis rats
- PMID: 30887626
- PMCID: PMC6790651
- DOI: 10.1111/nep.13587
Protective effects and mechanisms of omega-3 polyunsaturated fatty acid on intestinal injury and macrophage polarization in peritoneal dialysis rats
Abstract
Aim: This study was conducted to investigate the chronic injury of peritoneal glucose injection on the peritoneum and intestine and the protective effects of omega-3 polyunsaturated fatty acid (ω-3PUFA) during peritoneal dialysis (PD).
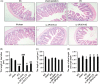
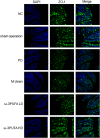
Methods: Peritoneal dialysis animal models were established by intraperitoneal injection of 4.25% glucose for 28 days. Protein expression in ileum and peritoneum was measured by immunofloresence and immunohistochemistry. Protein expression in macrophages was measured by Western blot. Fibrosis was analyzed by Masson staining.
Results: Peritoneal dialysis significantly increased the structural injury and decreased junction-related protein ZO-1 and occludin expression in ileum, the expression of proteins relating to the activation of M2 (Erg2, IRF4), but not M1 (CD38, IRF5) macrophages. PD significantly increased the expression of TGF-β1, VEGF and ALK5 protein in peritoneal tissues. PD significantly increased fibrosis (Masson staining) and the expression of fibroblast marker α-SMA in peritoneal tissues. Injection of macrophage clean reagent and ω-3PUFA significantly inhibited M2 activation, and decreased Masson staining, α-SMA, TGF-β1, VEGF and ALK5 protein expression in peritoneal tissues in PD treated rats. ω-3PUFA injection significantly decreased PD-induced injury in ileum and normalized the expression of ZO-1 and occludin in the ileum of PD rats.
Conclusion: Omega-3 fatty acids can provide a protective role on PD-induced peritoneal fibrosis and injury of the intestine.
Keywords: fibrosis; intestinal injury; macrophage polarization; omega-3 polyunsaturated fatty acid; peritoneal dialysis.
© 2019 The Authors Nephrology published by John Wiley & Sons Australia, Ltd on behalf of Asian Pacific Society of Nephrology.
Figures







Similar articles
-
[High glucose dialysate enhances peritoneal fibrosis through upregulating glucose transporters GLUT1 and SGLT1].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016 May 25;45(6):598-606. doi: 10.3785/j.issn.1008-9292.2016.11.07. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016. PMID: 28247603 Free PMC article. Chinese.
-
TGF-β1-VEGF-A pathway induces neoangiogenesis with peritoneal fibrosis in patients undergoing peritoneal dialysis.Am J Physiol Renal Physiol. 2018 Feb 1;314(2):F167-F180. doi: 10.1152/ajprenal.00052.2017. Epub 2017 Oct 4. Am J Physiol Renal Physiol. 2018. PMID: 28978530
-
The aldosterone receptor antagonist spironolactone prevents peritoneal inflammation and fibrosis.Lab Invest. 2014 Aug;94(8):839-50. doi: 10.1038/labinvest.2014.69. Epub 2014 May 26. Lab Invest. 2014. PMID: 24862968
-
Complement system activation and peritoneal membrane alterations: Culprit or innocent bystander?Perit Dial Int. 2020 Mar;40(2):115-123. doi: 10.1177/0896860819896242. Epub 2020 Jan 13. Perit Dial Int. 2020. PMID: 32063185 Review.
-
Peritoneal Membrane Preservation.Semin Nephrol. 2017 Jan;37(1):77-92. doi: 10.1016/j.semnephrol.2016.10.009. Semin Nephrol. 2017. PMID: 28153197 Review.
Cited by
-
Mechanisms underlying the involvement of peritoneal macrophages in the pathogenesis and novel therapeutic strategies for dialysis-induced peritoneal fibrosis.Front Immunol. 2024 Dec 19;15:1507265. doi: 10.3389/fimmu.2024.1507265. eCollection 2024. Front Immunol. 2024. PMID: 39749340 Free PMC article. Review.
-
IL-17A as a Potential Therapeutic Target for Patients on Peritoneal Dialysis.Biomolecules. 2020 Sep 24;10(10):1361. doi: 10.3390/biom10101361. Biomolecules. 2020. PMID: 32987705 Free PMC article. Review.
-
Astragaloside IV Alleviates Intestinal Barrier Dysfunction via the AKT-GSK3β-β-Catenin Pathway in Peritoneal Dialysis.Front Pharmacol. 2022 Apr 27;13:873150. doi: 10.3389/fphar.2022.873150. eCollection 2022. Front Pharmacol. 2022. PMID: 35571132 Free PMC article.
-
Omega-3 fatty acids impair miR-1-3p-dependent Notch3 down-regulation and alleviate sepsis-induced intestinal injury.Mol Med. 2022 Jan 28;28(1):9. doi: 10.1186/s10020-021-00425-w. Mol Med. 2022. PMID: 35090386 Free PMC article.
-
Potential of nutraceuticals in celiac disease.Tissue Barriers. 2025;13(2):2374628. doi: 10.1080/21688370.2024.2374628. Epub 2024 Jun 30. Tissue Barriers. 2025. PMID: 38944818 Free PMC article. Review.
References
-
- Tomino Y. Mechanisms and interventions in peritoneal fibrosis. Clin. Exp. Nephrol. 2012; 16: 109–14. - PubMed
-
- Zhou Q, Bajo MA, Del Peso G, Yu X, Selgas R. Preventing peritoneal membrane fibrosis in peritoneal dialysis patients. Kidney Int. 2016; 90: 515–24. - PubMed
-
- Raby AC, González‐Mateo GT, Williams A et al. Targeting toll‐like receptors with soluble toll‐like receptor 2 prevents peritoneal dialysis solution‐induced fibrosis. Kidney Int. 2018; 94: 346–62. - PubMed
-
- Onishi A, Morishita Y, Muto S, Kusano E. The mechanism of peritoneal fibrosis in peritoneal dialysis. J. Nephrol. Ther. 2011; S3: 002.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

