Re-biopsy and liquid biopsy for patients with non-small cell lung cancer after EGFR-tyrosine kinase inhibitor failure
- PMID: 30887673
- PMCID: PMC6449239
- DOI: 10.1111/1759-7714.13035
Re-biopsy and liquid biopsy for patients with non-small cell lung cancer after EGFR-tyrosine kinase inhibitor failure
Abstract
Background: Re-biopsy is important for exploring resistance mechanisms, especially for non-small cell lung cancer (NSCLC) patients who develop resistance to EGFR-tyrosine kinase inhibitors (TKIs). Liquid biopsy using circulating tumor DNA has come into use for this purpose. This retrospective study investigated the status of re-biopsy and liquid biopsy in NSCLC patients with EGFR mutations and evaluated their effect on clinical strategies and prognosis.
Methods: Five hundred fifty-five NSCLC patients with resistance to EGFR-TKIs were included and divided into three groups: re-biopsy, liquid biopsy, and no re-biopsy. Amplification refractory mutation system (ARMS) PCR or super ARMS PCR was used to detect EGFR mutations.
Results: Three hundred eight (55.5%) patients underwent re-biopsy; 45.5% (140/308) were positive for T790M. The most common re-biopsy procedure was computed tomography-guided percutaneous core needle biopsy (60.1%), followed by effusion drainage (29.5%) and superficial lymph node biopsy (6.5%). One hundred eighteen (21.3%) patients underwent liquid biopsy; the T790M detection rate was 41.5% (49/118.) Of the 308 patients who underwent re-biopsy, 69 were examined for EGFR mutations with plasma. The concordance rate of T790M detection between tissue and plasma was 66.7%. A statistical difference in further treatment after EGFR-TKI failure was observed among all groups (P = 0.014). Patients in the biopsy groups were more likely to receive third-generation EGFR-TKIs. Multivariate analysis showed that re-biopsy had a significant impact on overall survival (P < 0.001).
Conclusion: Re-biopsy plays a pivotal role in the management of patients with NSCLC and resistance to EGFR-TKIs. Liquid biopsy may be an alternative if difficulties performing re-biopsy exist.
Keywords: EGFR-TKI resistance; liquid biopsy; non-small cell lung cancer; re-biopsy.
© 2019 The Authors. Thoracic Cancer published by China Lung Oncology Group and John Wiley & Sons Australia, Ltd.
Figures
 ) CT‐guided PCNB, (
) CT‐guided PCNB, ( ) Effusion drainage, (
) Effusion drainage, ( ) TBNA/EBUS‐TBNA, (
) TBNA/EBUS‐TBNA, ( ) SLNB and (
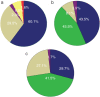
) SLNB and ( ) other metastasis biopsy. The distribution of results in patients who were tested for EGFR mutation via (b) tissue (c) or plasma. (
) other metastasis biopsy. The distribution of results in patients who were tested for EGFR mutation via (b) tissue (c) or plasma. ( ) Consistent with baseline, (
) Consistent with baseline, ( ) Mut plus T790M/T790M, (
) Mut plus T790M/T790M, ( ) Wild type, and (
) Wild type, and ( ) others. “Others” in (b) include three patients with small‐cell lung cancer, two with baseline mutations and c‐MET, one with an exon 20 insert, one with translation from 19del to L858R, one with KRAS and one with ALK. “Others” in (c) include one patient with an exon 20 insert and one with baseline mutations (L858R), S768I, and T790M. CT, computed tomography; EBUS, endobronchial ultrasonography; mut plus T790M, patients harbored a baseline mutation and a T790M mutation when re‐tested; PCNB, percutaneous core needle biopsy; SLNB, superficial lymph node biopsy; TBNA, transbronchial needle aspiration; T790M, patients only harbored a T790M mutation and the baseline sensitive mutation disappeared.
) others. “Others” in (b) include three patients with small‐cell lung cancer, two with baseline mutations and c‐MET, one with an exon 20 insert, one with translation from 19del to L858R, one with KRAS and one with ALK. “Others” in (c) include one patient with an exon 20 insert and one with baseline mutations (L858R), S768I, and T790M. CT, computed tomography; EBUS, endobronchial ultrasonography; mut plus T790M, patients harbored a baseline mutation and a T790M mutation when re‐tested; PCNB, percutaneous core needle biopsy; SLNB, superficial lymph node biopsy; TBNA, transbronchial needle aspiration; T790M, patients only harbored a T790M mutation and the baseline sensitive mutation disappeared.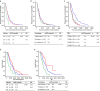
 ) PR, (
) PR, ( ) SD and (
) SD and ( ) PD, (b) patients who underwent re‐biopsy or not, if re‐biopsy (
) PD, (b) patients who underwent re‐biopsy or not, if re‐biopsy ( ) re‐biopsy, (
) re‐biopsy, ( ) liquid biopsy and (
) liquid biopsy and ( ) no biopsy and (c) patients divided into different groups according to whether T790M was positive and third‐generation TKIs (3‐TKIs) were administered, TBR (
) no biopsy and (c) patients divided into different groups according to whether T790M was positive and third‐generation TKIs (3‐TKIs) were administered, TBR ( ) T790M +3‐TKI+, (
) T790M +3‐TKI+, ( ) T790M +3‐TKI−, (
) T790M +3‐TKI−, ( ) T790M −3‐TKI+ and (
) T790M −3‐TKI+ and ( ) T790M −3‐TKI−. Kaplan–Meier estimates of the duration of overall survival (OS) of (d) patients who exhibited an objective response to EGFR‐TKIs, efficacy (
) T790M −3‐TKI−. Kaplan–Meier estimates of the duration of overall survival (OS) of (d) patients who exhibited an objective response to EGFR‐TKIs, efficacy ( ) PR, (
) PR, ( ) SD, (
) SD, ( ) PD, (
) PD, ( ) PR‐censored, (
) PR‐censored, ( ) SD‐censored and (
) SD‐censored and ( ) PD‐censored and (e) patients who underwent re‐biopsy or not, if re‐biopsy (
) PD‐censored and (e) patients who underwent re‐biopsy or not, if re‐biopsy ( ) re‐biopsy, (
) re‐biopsy, ( ) liquid biopsy, (
) liquid biopsy, ( ) no rebiopsy, (
) no rebiopsy, ( ) re‐biopsy‐censored, (
) re‐biopsy‐censored, ( ) liquid biopsy‐censored and (
) liquid biopsy‐censored and ( ) no rebiopsy‐censored. HR, hazard ratio; mOS, median OS; mPFS, median PFS; mut plus T790M, patients harbored a baseline mutation and a T790M mutation when re‐tested; PD, progressive disease; PR, partial response; SD, stable disease; T790M, patients only harbored a T790M mutation and the baseline sensitive mutation disappeared.
) no rebiopsy‐censored. HR, hazard ratio; mOS, median OS; mPFS, median PFS; mut plus T790M, patients harbored a baseline mutation and a T790M mutation when re‐tested; PD, progressive disease; PR, partial response; SD, stable disease; T790M, patients only harbored a T790M mutation and the baseline sensitive mutation disappeared.Similar articles
-
Next-generation sequencing of tissue and circulating tumor DNA: Resistance mechanisms to EGFR targeted therapy in a cohort of patients with advanced non-small cell lung cancer.Cancer Med. 2021 Jul;10(14):4697-4709. doi: 10.1002/cam4.3948. Epub 2021 Jun 25. Cancer Med. 2021. PMID: 34173341 Free PMC article.
-
The impact of the tumor shrinkage by initial EGFR inhibitors according to the detection of EGFR-T790M mutation in patients with non-small cell lung cancer harboring EGFR mutations.BMC Cancer. 2018 Dec 11;18(1):1241. doi: 10.1186/s12885-018-5153-4. BMC Cancer. 2018. PMID: 30537950 Free PMC article.
-
Impact of coexisting gene mutations in EGFR-mutated non-small cell lung cancer before treatment on EGFR T790M mutation status after EGFR-TKIs.Lung Cancer. 2020 Jan;139:28-34. doi: 10.1016/j.lungcan.2019.10.028. Epub 2019 Nov 3. Lung Cancer. 2020. PMID: 31710890
-
Understanding the Mechanisms of Resistance in EGFR-Positive NSCLC: From Tissue to Liquid Biopsy to Guide Treatment Strategy.Int J Mol Sci. 2019 Aug 14;20(16):3951. doi: 10.3390/ijms20163951. Int J Mol Sci. 2019. PMID: 31416192 Free PMC article. Review.
-
Dacomitinib in lung cancer: a "lost generation" EGFR tyrosine-kinase inhibitor from a bygone era?Drug Des Devel Ther. 2015 Oct 15;9:5641-53. doi: 10.2147/DDDT.S52787. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26508839 Free PMC article. Review.
Cited by
-
Optimizing ctDNA: An Updated Review of a Promising Clinical Tool for the Management of Uveal Melanoma.Cancers (Basel). 2024 Sep 1;16(17):3053. doi: 10.3390/cancers16173053. Cancers (Basel). 2024. PMID: 39272911 Free PMC article. Review.
-
The Feasibility of Interventional Pulmonology Methods for Detecting the T790M Mutation after the First or Second-Generation EGFR-TKI Resistance of Non-Small Cell Lung Cancer.Diagnostics (Basel). 2022 Dec 30;13(1):129. doi: 10.3390/diagnostics13010129. Diagnostics (Basel). 2022. PMID: 36611420 Free PMC article.
-
An EGFR T790M-mutated lung adenocarcinoma undergoing large-cell neuroendocrine carcinoma transformation after osimertinib therapy: a case report.J Med Case Rep. 2020 Aug 7;14(1):122. doi: 10.1186/s13256-020-02447-0. J Med Case Rep. 2020. PMID: 32762742 Free PMC article.
-
Tissue Adequacy and Safety of Percutaneous Transthoracic Needle Biopsy for Molecular Analysis in Non-Small Cell Lung Cancer: A Systematic Review and Meta-analysis.Korean J Radiol. 2021 Dec;22(12):2082-2093. doi: 10.3348/kjr.2021.0244. Epub 2021 Aug 31. Korean J Radiol. 2021. PMID: 34564960 Free PMC article.
-
Liquid and Tissue Biopsies for Lung Cancer: Algorithms and Perspectives.Cancers (Basel). 2024 Sep 29;16(19):3340. doi: 10.3390/cancers16193340. Cancers (Basel). 2024. PMID: 39409960 Free PMC article. Review.
References
-
- Chen W, Zheng R, Baade PD et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66 (2): 115–32. - PubMed
-
- Allemani C, Matsuda T, Di Carlo V et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet 2018; 391 (10125): 1023–75. - PMC - PubMed
-
- Dolly SO, Collins DC, Sundar R et al. Advances in the development of molecularly targeted agents in non‐small‐cell lung cancer. Drugs 2017; 77 (8): 813–27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

