Prospective study of the efficacy and utility of TP53 mutations in circulating tumor DNA as a non-invasive biomarker of treatment response monitoring in patients with high-grade serous ovarian carcinoma
- PMID: 30887755
- PMCID: PMC6424844
- DOI: 10.3802/jgo.2019.30.e32
Prospective study of the efficacy and utility of TP53 mutations in circulating tumor DNA as a non-invasive biomarker of treatment response monitoring in patients with high-grade serous ovarian carcinoma
Abstract
Objective: Somatic TP53 mutation (TP53mut) is a characteristic finding in high-grade serous ovarian cancer (HGSOC). The aim of this study was to assess the clinical efficacy and utility of TP53mut circulating tumor DNA (ctDNA) monitoring as a biomarker for managing HGSOC.
Methods: TP53muts were evaluated in patients who received primary treatment for suspected ovarian cancer at Asan Medical Center. In patients diagnosed with HGSOC and with TP53mut, ctDNA, cancer antigen 125 (CA 125), and computed tomography were followed up according to the treatment course.
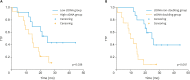
Results: Direct sequencing analysis of 103 tumor tissues from 61 HGSOC patients confirmed TP53muts in 41 patients (67.2%). All these patient-specific somatic mutations were detected in plasma cell-free DNA. The mean value of preoperative TP53 mutant allele count (TP53MAC) in stage III patients was 12.2 copies/μL and in stage IV patients was 45.3 copies/μL. TP53MAC was significantly reduced by treatment and there was no significant difference in the rate of decrease compared to CA 125 by the generalized linear mixed model. When patients were divided into a low TP53MAC group (<0.2 copies/μL) and a high TP53MAC group (≥0.2 copies/μL) based on the TP53MAC value at 3 months after the end of chemotherapy, there was a significant difference in time to progression between the two groups (p=0.038).
Conclusion: TP53mut ctDNA shows potential as a tumor-specific biomarker for treatment response monitoring in HGSOC. TP53mut ctDNA levels at 3 months post treatment has a significant prognostic utility than that of CA 125.
Keywords: DNA Mutational Analysis; Ovarian Cancer; Tumor Biomarkers; Tumor Suppressor Protein p53.
Copyright © 2019. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
Similar articles
-
Improving cell-free DNA detection in advanced-stage high-grade serous ovarian cancer using combined TP53 mutational status and copy number changes.Pathol Res Pract. 2025 Aug;272:156038. doi: 10.1016/j.prp.2025.156038. Epub 2025 May 31. Pathol Res Pract. 2025. PMID: 40482384
-
Exploratory Analysis of TP53 Mutations in Circulating Tumour DNA as Biomarkers of Treatment Response for Patients with Relapsed High-Grade Serous Ovarian Carcinoma: A Retrospective Study.PLoS Med. 2016 Dec 20;13(12):e1002198. doi: 10.1371/journal.pmed.1002198. eCollection 2016 Dec. PLoS Med. 2016. PMID: 27997533 Free PMC article.
-
Clonal Evolution of TP53 c.375+1G>A Mutation in Pre- and Post- Neo-Adjuvant Chemotherapy (NACT) Tumor Samples in High-Grade Serous Ovarian Cancer (HGSOC).Cells. 2019 Oct 1;8(10):1186. doi: 10.3390/cells8101186. Cells. 2019. PMID: 31581548 Free PMC article.
-
Liquid biopsy in ovarian cancer using circulating tumor DNA and cells: Ready for prime time?Cancer Lett. 2020 Jan 1;468:59-71. doi: 10.1016/j.canlet.2019.10.014. Epub 2019 Oct 11. Cancer Lett. 2020. PMID: 31610267 Review.
-
Circulating Tumour DNA for Ovarian Cancer Diagnosis and Treatment Monitoring: What Perspectives for Clinical Use?Int J Mol Sci. 2025 Feb 22;26(5):1889. doi: 10.3390/ijms26051889. Int J Mol Sci. 2025. PMID: 40076521 Free PMC article. Review.
Cited by
-
Potential value of circulating tumor DNA in gynecological tumors.Am J Transl Res. 2020 Jul 15;12(7):3225-3233. eCollection 2020. Am J Transl Res. 2020. PMID: 32774696 Free PMC article. Review.
-
ctDNA as an Objective Marker for Postoperative Residual Disease in Primary Advanced High-Grade Serous Ovarian Cancer.Cancers (Basel). 2025 Feb 25;17(5):786. doi: 10.3390/cancers17050786. Cancers (Basel). 2025. PMID: 40075633 Free PMC article.
-
Liquid biopsy in gynecological cancers: a translational framework from molecular insights to precision oncology and clinical practice.J Exp Clin Cancer Res. 2025 May 8;44(1):140. doi: 10.1186/s13046-025-03371-1. J Exp Clin Cancer Res. 2025. PMID: 40340939 Free PMC article. Review.
-
Sensitive circulating tumor DNA-based residual disease detection in epithelial ovarian cancer.Life Sci Alliance. 2024 Apr 5;7(6):e202402658. doi: 10.26508/lsa.202402658. Print 2024 Jun. Life Sci Alliance. 2024. PMID: 38580393 Free PMC article.
-
Role of Homologous Recombination Repair (HRR) Genes in Uterine Leiomyosarcomas: A Retrospective Analysis.Cancers (Basel). 2022 Apr 12;14(8):1934. doi: 10.3390/cancers14081934. Cancers (Basel). 2022. PMID: 35454841 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Thrall MM, DeLoia JA, Gallion H, Avril N. Clinical use of combined positron emission tomography and computed tomography (FDG-PET/CT) in recurrent ovarian cancer. Gynecol Oncol. 2007;105:17–22. - PubMed
-
- Niloff JM, Knapp RC, Lavin PT, Malkasian GD, Berek JS, Mortel R, et al. The CA 125 assay as a predictor of clinical recurrence in epithelial ovarian cancer. Am J Obstet Gynecol. 1986;155:56–60. - PubMed
-
- Högberg T, Kågedal B. Long-term follow-up of ovarian cancer with monthly determinations of serum CA 125. Gynecol Oncol. 1992;46:191–198. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

