Rituximab maintenance overcomes the negative prognostic factor of obesity in CLL: Subgroup analysis of the international randomized AGMT CLL-8a mabtenance trial
- PMID: 30888118
- PMCID: PMC6488104
- DOI: 10.1002/cam4.1980
Rituximab maintenance overcomes the negative prognostic factor of obesity in CLL: Subgroup analysis of the international randomized AGMT CLL-8a mabtenance trial
Abstract
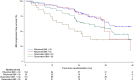
No data are available regarding obesity and outcome in Chronic Lymphocytic Leukemia (CLL). We analyzed 263 patients from the AGMT CLL-8a Mabtenance trial for the impact of obesity. The trial included patients after rituximab-containing induction treatment in first or second line that had achieved at least a PR. A randomization to rituximab maintenance treatment (375 mg/m2 q3 months for 2 years) vs observation was performed. In this cohort 22% of the patients (58/263) were classified as obese. The baseline response to induction treatment was inferior in obese patients with a lower CR rate (43.1% vs 60.5% in obese vs non-obese, P = 0.018) and with a lower rate of patients achieving MRD negativity after chemoimmunotherapy induction treatment (19.6% vs 35.8%, P = 0.02). The PFS outcome of obese patients was significantly worse in the observation group of the trial (24 vs 39 months median PFS, P = 0.03). However, in the rituximab maintenance group the outcome for obese vs non-obese was not different (P = 0.4). In summary, obesity was overall associated with a worse outcome of chemoimmunotherapy induction. However, rituximab maintenance treatment seems to be able to overcome this negative effect.
Keywords: BMI; CLL; maintenance; obesity; rituximab.
© 2019 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
SB receives support from Roche. AE receives grants and personal fees from Roche. RG receives grants and personal fees from Roche and Takeda, grants from Celgene and Novartis and personal fees from BMS and Amgen. MH receives personal fees from the Czech Lymphoma Study Group, and other support from Roche. TK receives other support from Roche. UJ receives grants and personal fees from Roche. AK receives personal fees from AGMT, Hoffmann‐La Roche, the Central European Society for Anticancer Drug Research and Arbeitsgemeinschaft Internistische Onkologie. JM receives other support from Roche, GlaxoSmithKline and Novartis. HO receives personal fees from Sanofi‐Aventis, Amgen and Celgene. PO receives grants from Roche, nonfinancial support from Janssen and Roche, and personal fees from GlaxoSmithKline and Gilead. AP receives personal fees from Roche. TP receives personal fees from Roche. LS receives personal fees from Abbvie, and personal fees and nonfinancial support from Gilead, Janssen and Roche. MS receives grants and personal fees from Roche. JA, PA, CB, MD, EF, LG, MG, EK, ML, EM, GM, Te.M., Th.M., TN, LP, MP, SP, JR, ES, OS, DV, LW and AZ declare no competing interests.
Figures
References
-
- Muller C, Murawski N, Wiesen M, et al. The role of sex and weight on rituximab clearance and serum elimination half‐life in elderly patients with DLBCL. Blood. 2012;119(14):3276‐3284. - PubMed
-
- Greil R, Obrtlíková P, Smolej L, et al. Rituximab maintenance versus observation alone in patients with chronic lymphocytic leukaemia who respond to first‐line or second‐line rituximab‐containing chemoimmunotherapy: final results of the AGMT CLL‐8a Mabtenance randomised trial. Lancet Haematol. 2016;3(7):e317‐e329. - PubMed
-
- Pfreundschuh M, Muller C, Zeynalova S, et al. Suboptimal dosing of rituximab in male and female patients with DLBCL. Blood. 2014;123(5):640‐646. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

