Pharmacological Treatment for Non-alcoholic Fatty Liver Disease
- PMID: 30888594
- PMCID: PMC6824365
- DOI: 10.1007/s12325-019-00898-6
Pharmacological Treatment for Non-alcoholic Fatty Liver Disease
Abstract
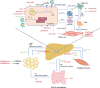
Non-alcoholic fatty liver disease (NAFLD) has become the most frequently encountered chronic liver disease. NAFLD is associated with increased liver-related morbidity and mortality, but also contributes to cardiovascular disease, diabetes and non-liver-related malignancy. Non-alcoholic steatohepatitis (NASH) is considered the more severe subtype of NAFLD that drives most of these adverse outcomes. Lifestyle modification and associated weight loss can improve NASH but are not always sufficient and sustained results are difficult to obtain. There is hence an urgent need for pharmacological treatment. In this review we discuss some of the concepts and challenges in the development of pharmacological treatment. We also briefly summarise what can be achieved with some of the drugs that are currently available for other indications but have demonstrated benefit in the treatment of NASH. Finally we present an overview of some of the main drugs or types of drugs, mainly based on their mode of action, that are now being developed specifically to treat NASH and that might soon result in the availability of drugs licensed for NASH.
Keywords: Cardiovascular disease; Clinical trials; Diabetes; Endpoints; Hepatology; Non-alcoholic steatohepatitis; Pharmacological treatment.
Figures
References
-
- Verrijken A, Francque S, Van Gaal L. The metabolic syndrome and the liver. Acta Gastroenterol Belg. 2008;71(1):48–49. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

