Randomized, Double-Blind, Placebo-Controlled Trial of Intraarticular Trans-Capsaicin for Pain Associated With Osteoarthritis of the Knee
- PMID: 30888737
- PMCID: PMC6772016
- DOI: 10.1002/art.40894
Randomized, Double-Blind, Placebo-Controlled Trial of Intraarticular Trans-Capsaicin for Pain Associated With Osteoarthritis of the Knee
Abstract
Objective: To assess the efficacy and safety of high-purity synthetic trans-capsaicin (CNTX-4975) in patients with chronic moderate-to-severe osteoarthritis (OA)-associated knee pain.
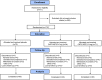
Methods: In this phase II multicenter double-blind study, patients ages 45-80 years who had stable knee OA were randomized in a 2:1:2 ratio to receive a single intraarticular injection of placebo, CNTX-4975 0.5 mg, or CNTX-4975 1.0 mg. The primary efficacy end point was area under the curve (AUC) for change from baseline in daily Western Ontario and McMaster Universities Osteoarthritis Index pain with walking score (range 0-10, 0 = none and 10 = extreme) through week 12. Secondary efficacy end points included a similar AUC analysis of outcomes in patients treated with CNTX-4975 0.5 mg, and evaluations extending to 24 weeks.
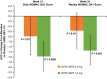
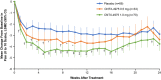
Results: Efficacy was evaluated in 172 patients (placebo group, n = 69; CNTX-4975 0.5 mg group, n = 33; CNTX-4975 1.0 mg group, n = 70). At week 12, greater decreases in the AUC for the pain score were observed with CNTX-4975 in the 0.5 mg and 1.0 mg groups versus placebo (0.5 mg group least squares mean difference [LSMD] -0.79, P = 0.0740; 1.0 mg group LSMD -1.6, P < 0.0001). Significant improvements were maintained at week 24 in the 1.0 mg group (LSMD -1.4, P = 0.0002). Treatment-emergent adverse events were similar in the placebo and CNTX-4975 1.0 mg groups.
Conclusion: In this study, CNTX-4975 provided dose-dependent improvement in knee OA-associated pain. CNTX-4975 1.0 mg produced a significant decrease in OA knee pain through 24 weeks; CNTX-4975 0.5 mg significantly improved pain at 12 weeks, but the effect was not evident at 24 weeks.
Trial registration: ClinicalTrials.gov NCT02558439.
© 2019 Centrexion Therapeutics Corporation. Arthritis & Rheumatology published by Wiley Periodicals, Inc. on behalf of American College of Rheumatology.
Figures
Comment in
-
Trans-capsaicin for Pain Reduction and Improvement in Function in Patients With Knee Osteoarthritis: Comment on the Article by Stevens et al.Arthritis Rheumatol. 2020 Aug;72(8):1404-1405. doi: 10.1002/art.41286. Epub 2020 Jun 4. Arthritis Rheumatol. 2020. PMID: 32293119 No abstract available.
-
Reply.Arthritis Rheumatol. 2020 Aug;72(8):1405-1406. doi: 10.1002/art.41287. Epub 2020 May 31. Arthritis Rheumatol. 2020. PMID: 32301590 No abstract available.
References
-
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma‐Zeinstra SM, et al. OARSI guidelines for the non‐surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. - PubMed
-
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–74. - PubMed
-
- Rutjes AW, Jüni P, da Costa BR, Trelle S, Nüesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta‐analysis. Ann Intern Med 2012;157:180–91. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

