Chronic gestational hypoxia accelerates ovarian aging and lowers ovarian reserve in next-generation adult rats
- PMID: 30888848
- PMCID: PMC6529349
- DOI: 10.1096/fj.201802772R
Chronic gestational hypoxia accelerates ovarian aging and lowers ovarian reserve in next-generation adult rats
Abstract
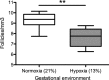
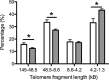
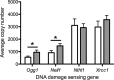
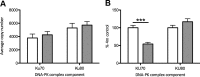
Chronic fetal hypoxia is a common complication observed in human pregnancy, impacting pregnancies across global contexts. Exposure to chronic intrauterine hypoxia has major short- and long-term consequences for offspring health. However, the impact of chronic gestational hypoxia on female reproductive system development is unknown. We aimed to understand the impact of exposure to chronic fetal hypoxia on the developing female reproductive system. Wistar rat dams underwent normoxia (21%) or hypoxia (13%) during pregnancy. Postnatally, all female offspring were maintained in normoxic conditions into early adulthood. Female rats exposed to chronic gestational hypoxia (13%) during their intrauterine development had decreased ovarian primordial follicular reserve compared to controls (P < 0.05). Adult females who had been exposed to chronic fetal hypoxia had significantly reduced somatic ovarian telomere length (P < 0.05) and reduced ovarian protein expression of KU70, a critical component of the DNA-activated protein kinase repair complex (P < 0.01). Gene expression of NADPH oxidase 2-mediated oxidative stress markers was increased (P < 0.05). Exposure to chronic hypoxia during fetal development leads to accelerated aging of the somatic ovary and decreased ovarian reserve in adulthood. Ovarian aging is highly sensitive to gestational hypoxia, with implications for future fertility in next-generation offspring of high-risk pregnancies.-Aiken, C. E., Tarry-Adkins, J. L., Spiroski, A.-M., Nuzzo, A. M., Ashmore, T. J., Rolfo, A., Sutherland, M. J., Camm, E. J., Giussani, D. A., Ozanne, S. E. Chronic gestational hypoxia accelerates ovarian aging and lowers ovarian reserve in next-generation adult rats.
Keywords: developmental programming; fetal hypoxia; follicles; ovary; reproductive aging.
Conflict of interest statement
C.E.A. was supported by a Grant from the Addenbrooke’s Charitable Trust (ACT; RG94137) and by an Issac Newton Trust/Wellcome Trust ISSF/ University of Cambridge Joint Research Grant. S.E.O. is supported by the Medical Research Council (MC_UU_12012/4). D.A.G. is supported by The British Heart Foundation (PG/14/5/30546). The authors declare no conflicts of interest.
Figures
References
-
- Giussani D. A., Davidge S. T. (2013) Developmental programming of cardiovascular disease by prenatal hypoxia. J. Dev. Orig. Health Dis. 4, 328–337 - PubMed
-
- Richardson S. J., Nelson J. F. (1990) Follicular depletion during the menopausal transition. Ann. N. Y. Acad. Sci. 592, 13–20; discussion 44–51 - PubMed

