An initial genetic analysis of gemcitabine-induced high-grade neutropenia in pancreatic cancer patients in CALGB 80303 (Alliance)
- PMID: 30889042
- PMCID: PMC6636684
- DOI: 10.1097/FPC.0000000000000375
An initial genetic analysis of gemcitabine-induced high-grade neutropenia in pancreatic cancer patients in CALGB 80303 (Alliance)
Abstract
Objectives: One of the standard of care regimens for advanced pancreatic cancer is gemcitabine-based chemotherapy. The efficacy of gemcitabine is limited by dose-limiting hematologic toxicities especially neutropenia. Uncovering the variability of these toxicities attributed to germline DNA variation is of great importance.
Patients and methods: CALGB 80303 was a randomized study in advanced pancreatic cancer patients treated with gemcitabine with or without bevacizumab. The study protocol included genotyping of genes of gemcitabine disposition (CDA, DCTD, SLC29A1, SLC28A1, and SLC29A2), as well as a genome-wide analysis. The clinical phenotype was time to early high-grade neutropenia event accounting for progression or death or other treatment-terminating adverse events as competing for informative events. The inference was carried out on the basis of the association between genotype and cause-specific hazard of a neutropenic event.
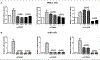
Results: The primary analyses were carried out on the basis of 294 genetically estimated European pancreatic cancer patients. For CDA rs2072671 (A>C), AC and CC patients had a lower risk of neutropenia than AA patients (P=0.01, hazard ratio: 0.61, 95% confidence interval: 0.41-0.89). For SLC28A1 rs3825876 (G>A), AA patients have a higher risk of neutropenia than GA and GG patients (P=0.02, hazard ratio: 1.51, 95% confidence interval: 1.06-2.16). CDA rs2072671 was associated with increased mRNA expression in whole blood in three studies (P=2.7e-14, 6.61e-62, and 9.70e-65). In the genome-wide analysis, variants in TGFB2 were among the top hits (lowest P=1.62e-06) but had no effect in luciferase assays.
Conclusion: This is the first genetic analysis of gemcitabine-induced neutropenia using a competing risk model in a prospective randomized clinical study has proposed a potentially novel mechanism of the protective effect of the CDA rs2072671 variant. Further confirmation is needed.
Figures



References
-
- Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28(22):3617–22. - PMC - PubMed
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. - PubMed
-
- Therneau TM, Grambsch PM (2000). Modeling Survival Data: Extending the Cox Model. New York: Springer.
-
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Vienna, Austria: [cited 2018]. Available from: www.R-project.org.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

