Contact dermatitis due to transdermal therapeutic systems: a clinical update
- PMID: 30889148
- PMCID: PMC6502158
- DOI: 10.23750/abm.v90i1.6563
Contact dermatitis due to transdermal therapeutic systems: a clinical update
Abstract
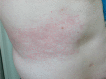
Transdermal therapeutic systems (TTS) have become a popular method of drug delivery because they allow drugs to be delivered in a rate-controlled manner, avoiding first-pass metabolism and the fluctuating plasma concentrations encountered with oral medications. Unfortunately, TTS may provoke adverse skin reactions as irritating contact dermatitis and allergic contact dermatitis: TTS seem to be ideally suited to produce sensitization because they cause occlusion, irritation, due to the repeated placement of the allergen in the same skin location. Since TTS consist of an adhesive, an active pharmaceutical drug and enhancing agents, sensitization may develop owing to one of these three agents. The purpose of this manuscript is to review known responsible allergens of contact dermatitis due to TTS.
Figures

References
-
- Subedi RK, Oh SY, Chun M-K, Choi H-K. Recent avances in transdermal drug delivery. Arch Pharm Res. 2010;33:339. - PubMed
-
- Ale I, Lachapelle J, Maibach H. Skin tolerability associated with transdermal drug delivery systems: an overview. Adv Ther. 2009;26:920–35. - PubMed
-
- Warshaw EM, Paller AS, Fowler JF, Zirwas MJ. Practical management of cutaneous reactions to the methylphenidate patch: recommendations from a Dermatology Expert Panel Consensus Meeting. Clin Ther. 2008;30:326–37. - PubMed
-
- Perez-Calderon R, Gonzalo-Garijo MA, Rodriguez-Nevado I. Generalized allergic contact dermatitis from nitroglycerin in a transdermal therapeutic system. Contact Dermatitis. 2002;46:303. - PubMed
-
- Machet L, Martin L, Toledano C, et al. Allergic contact dermatitis from nitroglycerin contained in 2 transdermal systems. Dermatology. 1999;198:106. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

