An ABCA4 loss-of-function mutation causes a canine form of Stargardt disease
- PMID: 30889179
- PMCID: PMC6424408
- DOI: 10.1371/journal.pgen.1007873
An ABCA4 loss-of-function mutation causes a canine form of Stargardt disease
Abstract
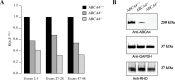
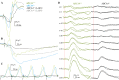
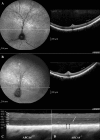
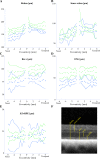
Autosomal recessive retinal degenerative diseases cause visual impairment and blindness in both humans and dogs. Currently, no standard treatment is available, but pioneering gene therapy-based canine models have been instrumental for clinical trials in humans. To study a novel form of retinal degeneration in Labrador retriever dogs with clinical signs indicating cone and rod degeneration, we used whole-genome sequencing of an affected sib-pair and their unaffected parents. A frameshift insertion in the ATP binding cassette subfamily A member 4 (ABCA4) gene (c.4176insC), leading to a premature stop codon in exon 28 (p.F1393Lfs*1395), was identified. In contrast to unaffected dogs, no full-length ABCA4 protein was detected in the retina of an affected dog. The ABCA4 gene encodes a membrane transporter protein localized in the outer segments of rod and cone photoreceptors. In humans, the ABCA4 gene is associated with Stargardt disease (STGD), an autosomal recessive retinal degeneration leading to central visual impairment. A hallmark of STGD is the accumulation of lipofuscin deposits in the retinal pigment epithelium (RPE). The discovery of a canine homozygous ABCA4 loss-of-function mutation may advance the development of dog as a large animal model for human STGD.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: A patent application (US No. 62/662,362) has been filed by the following authors and inventors, TB, GA, BE and SM. CM and SR are affiliated with a diagnostic lab marketing genetic tests for dogs. TB, GA and SM are affiliated with a university department that provides genetic testing for animals.
Figures