The protective effect of non-invasive low intensity pulsed electric field and fucoidan in preventing oxidative stress-induced motor neuron death via ROCK/Akt pathway
- PMID: 30889218
- PMCID: PMC6424404
- DOI: 10.1371/journal.pone.0214100
The protective effect of non-invasive low intensity pulsed electric field and fucoidan in preventing oxidative stress-induced motor neuron death via ROCK/Akt pathway
Abstract
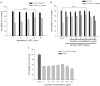
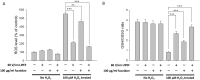
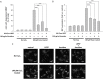
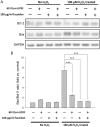
With the expansion of the aged population, it is predicted that neurodegenerative diseases (NDDs) will become a major threat to public health worldwide. However, existing therapies can control the symptoms of the diseases at best, rather than offering a fundamental cure. As for the complex pathogenesis, clinical and preclinical researches have indicated that oxidative stress, a central role in neuronal degeneration, is a possible therapeutic target in the development of novel remedies. In this study, the motor neuron-like cell line NSC-34 was employed as an experimental model in probing the effects induced by the combination of non-invasive low intensity pulsed electric field (LIPEF) and fucoidan on the H2O2-induced neuron damage. It was found that single treatment of the LIPEF could protect the NSC-34 cells from oxidative stress, and the protective effect was enhanced by combining the LIPEF and fucoidan. Notably, it was observed that single treatment of the LIPEF obviously suppressed the H2O2-enhanced expression of ROCK protein and increased the phosphorylation of Akt in the H2O2-treated NSC-34 cells. Moreover, the LIPEF can be easily modified to concentrate on a specific area. Accordingly, this technique can be used as an advanced remedy for ROCK inhibition without the drawback of drug metabolism. Therefore, we suggest the LIPEF would be a promising strategy as a treatment for motor neurodegeneration and warrant further probe into its potential in treating other neuronal degenerations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Protection of high-frequency low-intensity pulsed electric fields and brain-derived neurotrophic factor for SH-SY5Y cells against hydrogen peroxide-induced cell damage.Medicine (Baltimore). 2023 Aug 4;102(31):e34460. doi: 10.1097/MD.0000000000034460. Medicine (Baltimore). 2023. PMID: 37543811 Free PMC article.
-
Effect of high-frequency low-intensity pulsed electric field on protecting SH-SY5Y cells against hydrogen peroxide and β-amyloid-induced cell injury via ERK pathway.PLoS One. 2021 Apr 26;16(4):e0250491. doi: 10.1371/journal.pone.0250491. eCollection 2021. PLoS One. 2021. PMID: 33901243 Free PMC article.
-
VEGF-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death.Brain Res Mol Brain Res. 2003 Mar 17;111(1-2):155-64. doi: 10.1016/s0169-328x(03)00025-1. Brain Res Mol Brain Res. 2003. PMID: 12654515
-
Selenizing astragalus polysaccharide attenuates PCV2 replication promotion caused by oxidative stress through autophagy inhibition via PI3K/AKT activation.Int J Biol Macromol. 2018 Mar;108:350-359. doi: 10.1016/j.ijbiomac.2017.12.010. Epub 2017 Dec 5. Int J Biol Macromol. 2018. PMID: 29217185
-
Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities.Neuron. 2017 Mar 8;93(5):1015-1034. doi: 10.1016/j.neuron.2017.01.022. Neuron. 2017. PMID: 28279350 Review.
Cited by
-
Biological Potential, Gastrointestinal Digestion, Absorption, and Bioavailability of Algae-Derived Compounds with Neuroprotective Activity: A Comprehensive Review.Mar Drugs. 2022 May 28;20(6):362. doi: 10.3390/md20060362. Mar Drugs. 2022. PMID: 35736165 Free PMC article. Review.
-
Protection of high-frequency low-intensity pulsed electric fields and brain-derived neurotrophic factor for SH-SY5Y cells against hydrogen peroxide-induced cell damage.Medicine (Baltimore). 2023 Aug 4;102(31):e34460. doi: 10.1097/MD.0000000000034460. Medicine (Baltimore). 2023. PMID: 37543811 Free PMC article.
-
Acute Treatment with Fucoidan Ameliorates Traumatic Brain Injury-Induced Neurological Damages and Memory Deficits in Rats: Role of BBB Integrity, Microglial Activity, Neuroinflammation, and Oxidative Stress.Mol Neurobiol. 2025 May;62(5):5990-6013. doi: 10.1007/s12035-024-04668-6. Epub 2024 Dec 18. Mol Neurobiol. 2025. PMID: 39692820
-
Application of Marine Natural Products against Alzheimer's Disease: Past, Present and Future.Mar Drugs. 2023 Jan 5;21(1):43. doi: 10.3390/md21010043. Mar Drugs. 2023. PMID: 36662216 Free PMC article. Review.
-
Application of non-invasive low-intensity pulsed electric field with thermal cycling-hyperthermia for synergistically enhanced anticancer effect of chlorogenic acid on PANC-1 cells.PLoS One. 2020 Jan 29;15(1):e0222126. doi: 10.1371/journal.pone.0222126. eCollection 2020. PLoS One. 2020. PMID: 31995555 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

