CD40 Ligand-Modified Chimeric Antigen Receptor T Cells Enhance Antitumor Function by Eliciting an Endogenous Antitumor Response
- PMID: 30889381
- PMCID: PMC6428219
- DOI: 10.1016/j.ccell.2019.02.006
CD40 Ligand-Modified Chimeric Antigen Receptor T Cells Enhance Antitumor Function by Eliciting an Endogenous Antitumor Response
Abstract
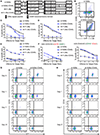
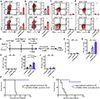
Chimeric antigen receptor (CAR) T cells provide great efficacy in B cell malignancies. However, improved CAR T cell therapies are still needed. Here, we engineered tumor-targeted CAR T cells to constitutively express the immune-stimulatory molecule CD40 ligand (CD40L) and explored efficacy in different mouse leukemia/lymphoma models. We observed that CD40L+ CAR T cells circumvent tumor immune escape via antigen loss through CD40/CD40L-mediated cytotoxicity and induction of a sustained, endogenous immune response. After adoptive cell transfer, the CD40L+ CAR T cells displayed superior antitumor efficacy, licensed antigen-presenting cells, enhanced recruitment of immune effectors, and mobilized endogenous tumor-recognizing T cells. These effects were absent in Cd40-/- mice and provide a rationale for the use of CD40L+ CAR T cells in cancer treatment.
Keywords: CAR T cells; CD40; CD40 ligand; antigen-presenting cells; chimeric antigen receptor; endogenous T cells; immunotherapy; leukemia; lymphoma.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures





References
-
- Banchereau J, and Steinman RM (1998). Dendritic cells and the control of immunity. Nature 392, 245–252. - PubMed
-
- Barbier L, Tay SS, McGuffog C, Triccas JA, McCaughan GW, Bowen DG, and Bertolino P (2012). Two lymph nodes draining the mouse liver are the preferential site of DC migration and T cell activation. J. Hepatol. - PubMed
-
- Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH, et al. (2013). A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin. Cancer Res. 19, 6286–6295. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

