Redox regulation of ER and mitochondrial Ca2+ signaling in cell survival and death
- PMID: 30889512
- PMCID: PMC8409742
- DOI: 10.1016/j.ceca.2019.02.006
Redox regulation of ER and mitochondrial Ca2+ signaling in cell survival and death
Abstract
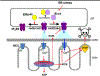
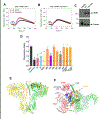
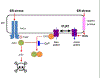
Physiological signaling by reactive oxygen species (ROS) and their pathophysiological role in cell death are well recognized. This review focuses on two ROS targets that are key to local Ca2+ signaling at the ER/mitochondrial interface - notably, inositol trisphosphate receptors (IP3Rs) and the mitochondrial calcium uniporter (MCU). Both transport systems are central to molecular mechanisms in cell survival and death. Methods for the measurement of the redox state of these proteins and for the detection of ROS nanodomains are described. Recent results on the redox regulation of these proteins are reviewed.
Keywords: Ca(2+); IP(3); IP(3) receptor; Mitochondrial calcium uniporter; Reactive oxygen species; Redox regulation.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Figures

References
-
- Tajeddine N, How do reactive oxygen species and calcium trigger mitochondrial membrane permeabilisation?, Biochimica et biophysica acta, 1860 (2016) 1079–1088. - PubMed
-
- Joseph SK, Role of thiols in the structure and function of IP3 receptors, Current Topics Membrane transport, 66 (2010) 299–322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

